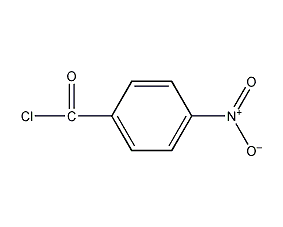
Structural formula
| Business number | 03EG |
|---|---|
| Molecular formula | C7H4ClNO3 |
| Molecular weight | 185.56 |
| label |
4-nitrobenzoyl chloride, p-nitrobenzoyl chloride, p-nitrobenzoyl chloride, Nitroxyl, P-nitrobenzoyl chloride, 4-nitrobenzoyl chloride p-nitrobenzoyl chloride, p-nitrobenzoyl chloride, 4-Nitrobenzoyl chloride, 4-Nitrobenzoic acid chloride, Labotest-Bb Lt00077260, P-Nitrobenzoyl Chloride, Nitrobenzoyl-4 chloride, 4-Nitro-benzoylchlorid, Benzoyl chloride, 4-nitro-, Benzoyl chloride, p-nitro-, catalyst, additives, chemical additives |
Numbering system
CAS number:122-04-3
MDL number:MFCD00007345
EINECS number:204-517-4
RTECS number:DM6651000
BRN number:473192
PubChem number:24886509
Physical property data
1. Properties: Bright yellow needle-like crystals, with a pungent odor, easy to deliquesce, and decompose when exposed to water and alcohol.
2. Melting point (℃): 72~75℃
3. Boiling point (ºC): 205℃/14kPa, 154℃/1.5kPa
4 . Solubility: Soluble in ether
Toxicological data
1. Acute toxicity: Rat oral LD50: 5600mg/kg
Mouse oral LC50: 3440mg/kg
Rabbit oral LD50: 4750mg/kg
p>
2. Other multiple dose toxicity: Oral TDLO in rats: 16800mg/kg/30D-I
Unknown mammal inhalation TCLO: 11830ug/m3/ 4H/17W-I
3. Reproductive toxicity: Unknown mammal (male, before mating) inhalation TCLO: 11830ug/m3/4H/16W
4. Mutagenic toxicity: Salmonella microbial mutation test system: 3ug/plate
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 43.04
2. Molar volume (cm3/mol): 127.6
3. Isotonic specific volume (90.2K): 346.5
4. Surface tension (dyne/cm): 54.2
5. Dielectric constant:
6�� Dipole moment (10-24cm3):
7, Polarizability: 17.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 62.9
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 193
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Toxic! It is corrosive and can cause burns.
2. Irritating to the respiratory system.
Storage method
1. Storage
Storage in sealed and dry packaging.
Synthesis method
1. This product can be produced by using p-nitrobenzoic acid as raw material and chlorination with thionyl chloride, phosphorus oxychloride or phosgene. P-nitrobenzoic acid and phosphorus oxychloride are heated and refluxed for 24 hours, and phosphorus oxychloride is recovered under reduced pressure to generate p-nitrobenzoyl chloride. Or heat p-nitrobenzoic acid and thionyl chloride to 90°C, reflux for 30-40 hours, and then cool to precipitate p-nitrobenzoyl chloride crystals. The yield is 90%. By passing phosgene into molten p-nitrobenzoic acid and distilling the phosgenation reactant under reduced pressure, p-nitrobenzoyl chloride can also be obtained.
2.Place dry p-nitrobenzoic acid and phosphorus pentachloride in an oil bath and heat until the reaction is complete, then recover under reduced pressure Phosphorus oxychloride. Then, vacuum distillation is used to evaporate the residue to obtain the crude product. After melting the crude product, add carbon tetrachloride, filter while it is hot, and cool and crystallize to obtain the finished product.
The process equation is:
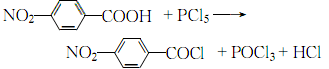
Purpose
1. Mainly used for the synthesis of cephazolin, procaine and folic acid. It is also widely used as an intermediate for dyes and pesticides.
2. Used in the determination of alcohols and phenols, dye manufacturing, pharmaceutical industry, and as an intermediate for color developers.

 微信扫一扫打赏
微信扫一扫打赏

