Background[1][2]
2-Benzyl-5-chloro-1,2,3,4-tetrahydro-[2,6]naphthyridine can be used as a pharmaceutical synthesis intermediate and an organic synthesis intermediate, mainly used in experiments Laboratory research and development process and chemical and pharmaceutical synthesis process.
Preparation[1]
2-Benzyl-5-chloro-1,2,3,4-tetrahydro-[2,6]naphthyridine is prepared as follows:
Step 1: N-ethoxy-3-methylpyridinium iodide:
Add 3-methylpyridine-N-oxide (50.00g, 458mmol) and anhydrous dichloromethane (90mL) to the round-bottomed flask. Ethyl iodide (715.57g, 4587mmol) was added and stirred at room temperature overnight. After the reaction was complete (TLC), the white solid formed was filtered and washed with ethyl acetate (2 × 50 mL) and dried to give the desired product (102 g, 86%).
Step 2: 3-methylpyridine-4-carbonitrile:
Add N-ethoxy-3-methylpyridinium iodide (74.00g, 536mmol), potassium carbonate (148.00g, 1072mmol) and water (350mL) to the round-bottomed flask. To this was slowly added a solution of sodium cyanide (49.9 g, 1018 mmol) in water (200 mL) over 30 minutes. The resulting reaction mixture was heated to 50°C for 2 hours. After the reaction was complete (TLC), the reaction mixture was extracted with ethyl acetate (3×500 mL). The combined organic layers were dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 28.5 g of product (a mixture of 2- and 4-isomers), which was used in the next step without further purification. [M+H]+=119.
Step 3: 3-(2-(dimethylamino)vinyl)pyridine-4-carbonitrile:
Add 3-methylpyridine-4-carbonitrile (52g, 440mmol), anhydrous N,N-dimethylformamide (100mL), N,N-dimethylformamide dimethyl into the round bottom flask. Acetal (314 g, 2644 mmol) and pyrrolidine (31 g, 440 mmol) were heated at 130 °C for 16 h under an inert atmosphere. After the reaction was complete (TLC), the reaction was treated with ice-cold water (300 mL) and extracted with ethyl acetate (3 × 500 mL). The combined organic layers were dried over anhydrous sodium sulfate and the solvent was removed to give 72 g of the desired product (a mixture of 2- and 4-isomers), which was used in the next step without further purification. MS: 174[M+H]+=174.
Step 4: 2,6-Νaphthyridin-1-ol:
Add 3-(2-(dimethylamino)vinyl)pyridine-4-carbonitrile (40.00g, 231mmol) and ethanol (100mL) and 48% aq to the round-bottomed flask. HBr (277g) was added and the reaction mixture was refluxed for 16 hours. After the reaction was completed (TLC), the solvent was removed and the residue was filtered and washed with cold ethanol to obtain the desired product (40%).
Step 5: 6-benzyl-5,6,7,8-tetrahydro-2,6-naphthyrid-1-ol:
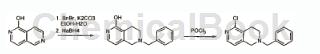
Add 2,6-naphthyrid-1-ol (17g, 116mmol), ethanol-water (1:2, 200mL) and benzyl bromide (99.52g, 582mmol) and potassium into the round-bottomed flask. Carbonate (8.5g, 61mmol) was added. The resulting reaction mixture was then refluxed for 3 hours. After the reaction was completed (TLC), the reaction mixture was cooled to 0°C, sodium borohydride (17.6g, 465mmol) was added in portions, and stirred at room temperature for 16 hours. Upon completion, the reaction was quenched with 6NHCl. The solid obtained was filtered, and the filtrate was alkalized with 10% NaOH solution to obtain a solid. The solid thus obtained was triturated with ethyl acetate and petroleum ether to obtain 9 g of the desired product. (32% yield).
Step 6: 2-Benzyl-5-chloro-1,2,3,4-tetrahydro-[2,6]naphthalene
Add 6-benzyl-5,6,7,8-tetrahydro-2,6-naphthyridin-1-ol (7 g, 29 mmol) and phosphorus oxychloride {Leonid Chemicals, India ; add 80 mL) and heat to reflux for 16 hours to monitor the reaction progress by TLC. After the reaction was completed, POCl3 was distilled and the residue was quenched with ice-cold water and then extracted with dichloromethane (3 × 100 mL). The combined organic layers were dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The residue was purified by column chromatography (methanol/dichloromethane, 5:95) to obtain the desired product 2-benzyl-5-chloro-1,2,3,4-tetrahydro-[2,6]di Azonaphthylene (yield 80%).
Main reference materials
[1] (WO2009011904) COMPOUNDS USEFUL AS FAAH MODULATORS AND USES THEREOF

 微信扫一扫打赏
微信扫一扫打赏

