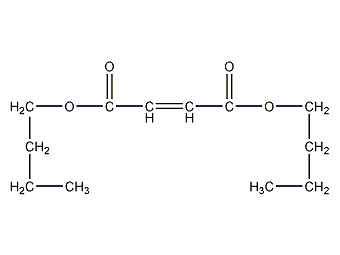
Structural formula
| Business number | 02SA |
|---|---|
| Molecular formula | C12H20O4 |
| Molecular weight | 228.28 |
| label |
dibutyl maleate, Dibutyl Maleate, CH3(CH2)3OCOCH=CHCOO(CH2)3CH3, plasticizer, pesticides, fungicides, anti-rust additives, aliphatic compounds |
Numbering system
CAS number:105-76-0
MDL number:MFCD00009447
EINECS number:203-328-4
RTECS number:ON0875000
BRN number:1726634
PubChem number:24893895
Physical property data
1. Properties: colorless oily liquid.
2. Density (g/mL, 25/4℃): 0.9907
3. Relative density (20℃, 4℃): 0.9964
4 . Melting point (ºC): < -80
5. Boiling point (ºC, normal pressure): 280.6
6. Viscosity (mPa·s, 25ºC): 4.76
7. Refractive index (25ºC): 1.4435
8. Flash point (ºC): 140.5
9. Relative density (25℃, 4℃): 0.937686.9
10. Refractive index at room temperature (n20): 1.4454
11. Liquid phase standard hot melt (J·mol-1·K-1): 432.5
12. Vapor pressure (kPa, 25ºC): 0.0021
13. Evaporation Heat (KJ/mol, 140~225ºC): 59.5
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Volume expansion coefficient (K-1, 20~30ºC): 0.00088
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Miscible with a variety of organic solvents, partially soluble in benzene and chloroform. Dissolves 0.05% in water at 25°C; water dissolves 0.05% in dibutyl maleate. It forms an azeotropic mixture with 98.4% water, with an azeotropic point of 99.9°C.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 500mgREACTION SEVERITY, slight reaction;
2. Acute toxicity: rat oral LD50: 3700mg/kg; mouse peritoneal Cavity LD50: 150mg/kg;
Rabbit skin contact LD50: 10mg/kg;
3. Mild irritation to skin and eyes.
Ecological data
None
Molecular structure data
1. Molar refractive index: 61.25
2. Molar volume (cm3/mol): 227.1
3. Isotonic specific volume (90.2K ): 546.2
4. Surface tension (dyne/cm): 33.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 24.28
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 10
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 52.6
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 209
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It is flammable. Pay attention to fire sources and prevent inhalation of vapor or contact with skin.
Storage method
Pay attention to fire sources and store in a cool place.
Synthesis method
1. Using maleic anhydride and butanol as raw materials, esterification is carried out under the catalysis of sulfuric acid. The reaction product is neutralized, washed with water, dealcoholized, distilled and filtered to obtain the finished product. Maleic anhydride reacts with methanol, ethanol, octanol, isooctyl alcohol or nonanol to obtain the corresponding dialkyl maleate. Raw material consumption quota: maleic anhydride (95%) 400kg/t, butanol 820kg/t.
Refining method: wash with dilute sodium carbonate solution, dry with anhydrous potassium carbonate or sodium sulfate and then distill under reduced pressure.
2. Preparation method:
In a reaction bottle equipped with a stirrer, water separator and thermometer, add 0.5g of sulfamic acid and maleic anhydride (2 ) (0.1mol), n-butanol 0.25mol, toluene 30mL. Heat and reflux under stirring, and the water generated during the reaction will continue to accumulate in the water separator until the amount of water no longer increases, which takes about 2 hours. Separate the water layer and continue heating to evaporate the toluene. The catalyst can precipitate at this time. After cooling, filter and distill under reduced pressure. Collect the fractions at 142℃/1.5kPa or 126℃/0.8kPa to obtain 20g of dibutyl maleate ① (1), with a yield of 92%. Note: ① Strongly acidic ion exchange resin can also be used as reaction catalyst, and the ester yield is about 90%. [1]
Purpose
1. Used as a raw material for synthetic resins and coatings, as well as impregnating agents, dispersants, lubricants, etc. in the petroleum industry, fabrics, plastics, and papermaking industries, and as polyvinyl chloride resins and methacrylic resins. of plasticizer. Maleic acid esters are used as intermediates in organic synthesis, mainly performing addition reactions on double bonds. For example, Diels-Alder reaction is carried out with butadiene, cyclopentadiene, etc., and compounds containing active hydrogen such as hydrogen, nitrile, thiol, and amine are added. The famous surfactant dialkyl succinate sulfonate, It is produced by the addition of sodium bisulfite. When other ammonia and amines are added to maleic acid esters, amidation (imidation) occurs due to aminolysis, which acts as an acylating agent. Diethyl maleate has a wide range of applications. It is also used as an intermediate for the production of highly effective pesticides such as Marathon and other pesticides and pharmaceuticals. It can also be used in the production of polymer compounds; dimethyl maleate, dibutyl maleate, dioctyl maleate Ester is an excellent plasticizer for PVC; it can be copolymerized with monomers such as vinyl chloride and vinyl acetate, and is used as coatings, adhesives, impregnating agents, dispersants, and lubricants for papermaking and fabrics; diisooctyl maleate Ester and dinonyl maleate are good petroleum pour point depressants. Also used in pesticides, fungicides, and anti-rust additives.
2. This product is an internal plasticizer that can be copolymerized with monomers such as vinyl chloride, vinyl acetate, styrene or acrylic esters. The copolymer is used in coatings, films, adhesives, and paper. Treatment agents, pigment fixing agents, impregnating agents, dispersants, lubricants, etc.

 微信扫一扫打赏
微信扫一扫打赏

