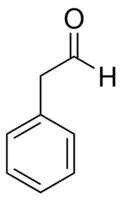
Structural formula
| Business number | 03FH |
|---|---|
| Molecular formula | C8H8O |
| Molecular weight | 120.15 |
| label |
α-Tolyaldehyde, a-Toluicaldehyde, Benzeneacetaldehyde, food additives, Flavor enhancer |
Numbering system
CAS number:122-78-1
MDL number:MFCD00006993
EINECS number:204-574-5
RTECS number:CY1420000
BRN number:385791
PubChem number:24901365
Physical property data
1. Properties: colorless or light yellow liquid with a hyacinth-like aroma and a fruity sweet aroma after dilution.
2. Density (g/mL, 25/4℃): 1.027
3. Refractive index (nD20): 1.5240-1.5320
4. Flash point (℃): 86
5. Melting point (℃): 33-34
6. Boiling point (ºC): 195
7. Solubility: Hardly soluble in water. Soluble in most organic solvents. Dissolve in 80% ethanol 1:2.
8. Stability: It is unstable in nature. It can polymerize and thicken when placed. It can be oxidized to phenylacetic acid and can also be reduced to phenylethanol. It can condense with alcohols (such as methanol and ethanol) to form acetal.
Toxicological data
1. Skin/eye irritation: Human skin standard Dreze eye dye test: 2%/48H
2. Acute toxicity: Rat oral LD50: 1550mg/kg
Mouse oral LD50: 3890mg/kg
Mouse inhalation LC50: 2gm/m3
Rabbit oral LD50:>200mg/kg
Rabbit skin LD50:>5gm/kg
Guinea pig oral LD50: 3890mg/kg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 40.46
2. Molar volume (cm3/mol): 135.8
3. Isotonic specific volume (90.2K): 328.7
4. Surface tension (dyne/cm): 34.2
5. Polarizability (10-24cm3): 16.04
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecular polar surface area 17.1
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 82.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Found in flue-cured tobacco leaves, burley tobacco leaves, and oriental tobacco leaves.
2. Naturally found in chicken, tomatoes, bread, rose oil, and citrus oil.
Storage method
Storage temperature 4ºC
Synthesis method
1. Mainly adopt synthetic method. It is obtained by reacting benzaldehyde and ethyl monochloroacetate in the presence of sodium ethoxide.
2. Tobacco: BU, 56; OR, 57; OR, 26; FC, BU, OR, 18; Synthesis: obtained by oxidation of phenylethyl alcohol or reduction of ethyl phenylacetate.
3. Preparation method:
Into a reaction bottle equipped with a stirrer, add 20g (81mmol) of 2-iodo-1-phenylethanol (2), 100mL of water, and 250mL of purified dioxane. While stirring, add dropwise a solution of 45g silver nitrate dissolved in 100mL water over 10 minutes. After the addition, continue to stir the reaction for 15 minutes, filter, and extract with petroleum ether. After the organic layer was washed with water, it was extracted twice with an aqueous solution containing 40g of sodium bisulfite, 100mL each time. The resulting precipitate was filtered, washed with water and pomegranate ether to obtain 28 g of phenylacetaldehyde sodium bisulfite adduct, with a yield of 52%. [1]
Purpose
1. Aldehyde synthetic fragrances. It is mainly used as a blending spice for hyacinth, lilac, rose, lily, narcissus, acacia, sweet pea, cyclamen and other floral flavors.
2. It can be condensed with methanol, ethanol, etc. to form acetal (can be used as a spice), which is used in the spice industry. Also used in baked goods, frozen dairy products, puddings.

 微信扫一扫打赏
微信扫一扫打赏

