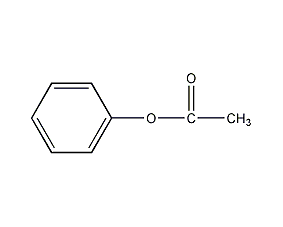
Structural formula
| Business number | 03FJ |
|---|---|
| Molecular formula | C8H8O2 |
| Molecular weight | 136.15 |
| label |
phenyl acetate, Acetic acid, phenyl ester, Acetoxybenzene, aromatic compounds |
Numbering system
CAS number:122-79-2
MDL number:MFCD00008699
EINECS number:204-575-0
RTECS number:AJ2800000
BRN number:636458
PubChem number:24901952
Physical property data
1. Properties: colorless liquid with strong refractive properties and phenol smell.
2. Relative density (g/mL, 20/4℃): 1.0780
3. Refractive index (20ºC): 1.5035
4. Flash point (℃): 76
5. Solubility: Miscible with ethanol, ether, chloroform and acetic acid, almost insoluble in water.
6. Boiling point (ºC): 195.7~196
7. Relative density (25℃, 4℃): 1.0685
8. Refractive index at room temperature ( n25): 1.5035
9. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4021.2
10. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -270.3
11. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3966.0
12. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -325.5
Toxicological data
1. Skin/eye irritation: Rabbit skin irritation test: 535mg is slightly irritating to the skin.
2. Acute toxicity: rat oral LD50: 1630uL/kg; rabbit skin LD50: 8mL/kg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 37.59
2. Molar volume (cm3/mol): 127.0
3. Isotonic specific volume (90.2K ): 308.7
4. Surface tension (dyne/cm): 34.8
5. Polarizability (10-24cm3): 14.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 114
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocentersAmount: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Covalent bonds Number of units: 1
Properties and stability
1. Flammable. It is easy to burn when exposed to open flames or high heat.
2. Exist in mainstream smoke.
3. Acute oral LD in rats501.63ML/kg
Storage method
Synthesis method
Obtained from the reaction of sodium phenolate and acetic anhydride. Add phenol to 15% sodium hydroxide solution, stir and dissolve to prepare a sodium phenolate solution, add acetic anhydride, and react at 30-40°C. The obtained reaction product was washed with water, 5% sodium hydroxide solution, and water in sequence, dried with calcium chloride, and then distilled to obtain the finished product. The reaction time required for this method is very short, and the yield is about 77%. Another operation method is to heat phenol and acetic anhydride together to boiling, reflux for 3 hours, and then wash with water, alkali, and water in sequence after cooling. After drying with anhydrous sodium sulfate, collect the 190-195°C fraction by distillation, preferably benzene acetate. ester. The yield is about 83%.
Purpose
Used as a solvent and an intermediate in organic synthesis, phenyl acetate undergoes translocation reaction to obtain hydroxyacetophenone, which is used to treat acute and chronic jaundice hepatitis and cholecystitis.

 微信扫一扫打赏
微信扫一扫打赏

