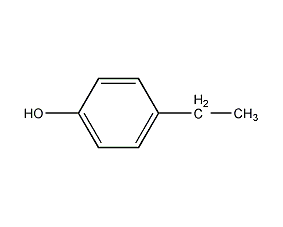
Structural formula
| Business number | 03G3 |
|---|---|
| Molecular formula | C8H10O |
| Molecular weight | 122.17 |
| label |
p-Ethylphenol, 1-hydroxy-4-ethylbenzene, p-Ethylphenol, 1-Hydroxy-4-ethylbenzene, 4-Ethyl-phenol, Plastic anti-aging agent, Surfactant, emulsifier |
Numbering system
CAS number:123-07-9
MDL number:MFCD00002393
EINECS number:204-598-6
RTECS number:SL4040000
BRN number:1363317
PubChem number:24862407
Physical property data
1. Properties: Colorless needle-like crystals
2. Melting point (℃): 46
3. Boiling point (℃): 219
4. Flash point (℃): 104
5. Solubility: Soluble in ethanol, ether, benzene and carbon disulfide, slightly soluble in water.
6. Relative density (20℃, 4℃): 1.0110
7. Refractive index (n25D): 1.5239
8. Refractive index at room temperature (n20): 1.5328d
9. Critical temperature (ºC): 443.25
10. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4443.1
11. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -144.1
12. Crystal phase standard combustion heat (Enthalpy) (kJ·mol-1): -4352.8
13. Crystal phase standard claims heat (enthalpy) (kJ·mol-1 ):-224.4
Toxicological data
1. Acute toxicity: mouse peritoneal cavity LD5O: 138mg/kg; 5. Toxicity LD50 (mg/kg): rat oral cavity 270
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 37.68
2. Molar volume (cm3/mol): 120.6
3. Isotonic specific volume (90.2K ): 298.8
4. Surface tension (dyne/cm): 37.6
5. Polarizability (10-24cm3): 14.93
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 72.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Found in flue-cured tobacco leaves and spicesTobacco leaves and mainstream smoke.
Storage method
None yet
Synthesis method
Preparation or source: (1) Extracted from coal tar. (2) It is produced by using phenol and ethylene as raw materials and phosphoric acid as catalyst at 200°C and 4.0-4.5 MPa. (3) It is produced by using 4-chloroethylbenzene as raw material and reacting with sodium hydroxide under the catalysis of copper powder. (4) It is prepared using phenol and 2-chloroethanol as raw materials and under the action of metallic sodium. The natural product is found in egg fruits. Darkens in color when exposed to light. (5): Tobacco: OR, 26; FC, 18.
Purpose
Used as raw materials for phenolic resin, rubber anti-aging agent, plastic anti-aging agent, surfactant, non-ionic emulsifier, synthetic spices and pesticides; edible spices; organic synthesis intermediates and chemical reagents.

 微信扫一扫打赏
微信扫一扫打赏

