Overview
P-Methoxyaniline is an important organic raw material that can be used to prepare a variety of pharmaceutical intermediates.
Apply[1-4]
1. Clausenine is a carbazole natural product with antibacterial activity. CN201410027161 reports a copper-catalyzed reaction of 6-diazo-3-methyl-2-cyclohexen-1-one and p-methoxyaniline to generate 2-(4-methoxyphenylamino)-5- Toluene; then through methylation and palladium-catalyzed C-C bond coupling, the natural product Clausenine can be obtained. The reaction route is as follows:
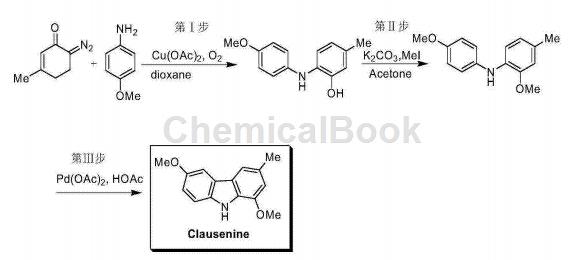
Follow the following steps:
(1) Add 6-diazo-3-methyl-2-cyclohexen-1-one, p-methoxyaniline, copper catalyst and solvent into the sealed tube, and seal it at 80 degrees Celsius. After 3 hours of reaction, 2-(4-methoxyphenylamino)-5-methylphenol can be obtained with high yield;
(2) Add 2-(4-methoxyphenylamino)-5-methylphenol, methyl iodide, potassium carbonate and acetone into the reaction bottle at room temperature, then raise the temperature to 60 degrees Celsius and react to obtain N- (2-methoxy-4-methylphenyl) p-methoxyaniline;
(3) Under nitrogen protection, add N-(2-methoxy-4-methylphenyl) p-methoxyaniline, palladium acetate and acetic acid to the sealed tube; then heat it up to 140 degrees Celsius to avoid light Clausenine can be obtained after 24 hours of reaction.
2. Omeprazole, 5-methoxy-2-[(4-methoxy-3, 5-dimethylpyridyl)methylsulfinyl]-1H-benzimidanol, It is the first proton pump inhibitor to be marketed in the world. It has a long-lasting inhibitory effect on gastrin, as well as gastric acid secretion caused by acetylcholine, histamine, food or vagus nerve stimulation. It is mainly used in ulcers, esophageal acid reflux disease and Treatment of gastrinomas and Helicobacter pylori infection. 4-Methoxy-2-nitroaniline is an important intermediate in the synthesis of omeprazole.
CN201611104733.5 provides a preparation method of 4-methoxy-2-nitroaniline. The technical solution adopted by the present invention is: place p-methoxyaniline in a 500mL beaker, quickly add acetic anhydride, stir vigorously, and ultrasonic for half an hour. At this time, the material is viscous. Take a small amount of dotted plate (petroleum ether: ethyl acetate). Ester = 1:1) The reaction is complete, add water, let it stand for crystallization, filter out the precipitated solid, wash the filter cake with water, and dry it to obtain off-white solid p-methoxyacetanilide.
Place p-methoxyacetanilide in a 500ml three-necked flask, add water, cool to 0~5°C in an ice bath, stir mechanically for half an hour, mix acid (concentrated sulfuric acid 30mL/concentrated nitric acid 30mL, molar ratio 1:5 ), cool to 0~5℃, add slowly, control to finish dropping within 1 hour, stir slowly, try not to let the splashed liquid stick to the wall of the bottle, the material in the bottle slowly changes from gray to dark yellow, and rises to room temperature. Stir for 1 hour, a large amount of insoluble light yellow solid appears, take a sampling spot (petroleum ether: ethyl acetate = 1:1), the reaction is complete, add water, continue stirring for half an hour, let it stand, filter, wash with water until neutral, and dry to obtain Light yellow solid o-nitro-p-methoxy-acetamidoaniline. Place 2-nitro-4-methoxy-acetamidoaniline in a 500mL round-bottomed flask, add NaOH aqueous solution at 60°C, stir for 1 hour, a red solid appears, and take a sampling spot (petroleum ether: ethyl acetate = 4 :1), the reaction is complete, cool carefully, and try to allow all the solids to precipitate. Filter out the solid, wash with water, and dry to obtain o-nitro-p-methoxyaniline as a red solid.
3. N-(5-methylfurfuryl)-p-methoxyaniline (CAS 95124-39-3; C13H15NO2; 2-Furanmethanamine, N-(4-methoxyphenyl)-5-methyl-) has certain Antibacterial function. CN201711076942.8 provides a method to prepare N-(5-methylfurfuryl)-p-methyl through direct reductive amination of 5-methylfurfural and p-methoxyaniline under a hydrogen atmosphere by adding a heterogeneous catalyst and using a simple one-pot method. Oxyaniline method. It includes the following steps: adding the heterogeneous hydrogenation catalyst, 5-methylfurfural, solvent, and p-methoxyaniline into the reaction vessel in sequence, in a hydrogen atmosphere of normal pressure to 2.0Mpa pressure, and at a reaction temperature of normal temperature to 100°C , stir, react for 0.2~30h, and separate the catalyst and product.
4. p-Methoxyphenyl isocyanate is an important intermediate for medicines, pesticides and polymer materials.
CN03124291.X provides a chemical compound of p-methoxyphenyl isocyanateIt is synthesized by a chemical synthesis method using p-methoxyaniline and bis(trichloromethyl)carbonate as raw materials in an organic solvent in the presence of a catalyst. The molar ratio of the input is p-methoxyaniline:bis(trichloromethyl). base) carbonate: the catalyst is 1:0.34~0.8:0.01-0.1; the amount of organic solvent is 3-12 times the mass of p-methoxyaniline; the reaction temperature is 20-180°C; the reaction time is 4~10h . The organic solvent may be benzene or toluene or xylene or chlorobenzene or dichlorobenzene or methylene chloride or chloroform or carbon tetrachloride or dichloroethane or tetrahydrofuran or epoxyhexane. The beneficial effects of the invention are: advanced process route, reasonable process conditions, the raw materials used avoid highly toxic phosgene and diphosgene, safe and reliable production, high reaction yield, generally above 93%, and low production cost. , equipment corrosion is small, three wastes are less and easy to handle, and it has great implementation value and social and economic benefits.
Main reference materials
[1] [China invention, China invention authorization] CN201410027161.X A method for synthesizing the natural product Clausenine
[2] [Chinese invention] CN201611104733.5 A preparation method of o-nitro-p-methoxyaniline
[3] [Chinese invention] CN201711076942.8 A one-pot method for preparing N-(5-methylfurfuryl)-p-methoxyaniline
[4] CN03124291.X A chemical synthesis method of p-methoxyphenyl isocyanate

 微信扫一扫打赏
微信扫一扫打赏

