chlorobenzene nitro compounds are extremely important organic chemical raw materials and are widely used in synthetic dyes, pigments, medicines, pesticides, rubber additives, engineering plastics, etc. the main industrial nitrification methods include: nitrification with dilute nitric acid, nitrification with concentrated nitric acid, nitrification with heterogeneous mixed acid, and nitrification in organic solvents. at present, due to the increase in domestic manufacturers year by year, old factories are tapping their potential and transforming to produce more products, the output has increased sharply, and the product market competition is fierce. the chlorobenzene nitration reaction is a mature chemical reaction, but there are obvious problems in the industrialization process, such as the production of a large amount of waste acid and unsatisfactory selectivity. for this reason, it is of positive significance to study the nitration reaction of aromatic hydrocarbons in microchannel reactors.
1 mechanism analysis
chlorobenzene nitration is a typical aromatic electrophilic substitution reaction. it is calculated using pm3 quantum chemistry method. the charge distribution of chlorobenzene is shown in figure 1. the para-position and ortho-position charges are more negative than the meta-position. therefore, to confirm the reaction process, nitration the acyl cation n02+ preferentially attacks the ortho and para positions, and the resulting product is mainly ortho and para positions.
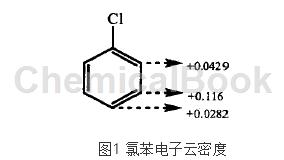
the chlorine atom is the ortho- and para-positioning group of passivated benzene ring, and the nitration situation of chlorobenzene is quite special. when n02+ attacks chlorobenzene, it first interacts with delocalized electrons and forms a π complex. at this time, no new bonds are formed. then, two electrons in the π system are taken away and a σ bond is formed, forming a σ complex. the σ complex is unstable and easily loses a proton, resulting in a delocalized closed covet system with lower energy. during the reaction, the process of conversion into σ complex and loss of protons proceeds rapidly, while the process of π complex is slower, which determines the rate of the entire reaction.
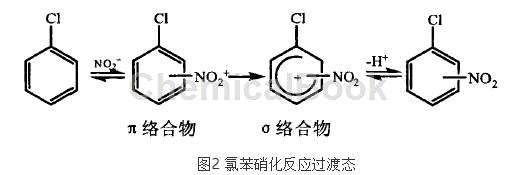
from the perspective of electronic effects, the stability of the transition state or σ complex during the nitration process is different, so the ease of substitution at each position is different. the chlorine atom has a strong attraction induction effect, and the electron cloud density of the benzene ring decreases.
therefore it is unfavorable for electrophilic substitution reactions. however, judging from the σ complex produced during the reaction, id and iid are particularly stable. therefore, although the electrophilic substitution reaction of chlorobenzene is more difficult to carry out than benzene, it still mainly occurs at the ortho and para positions of the chlorine atom.
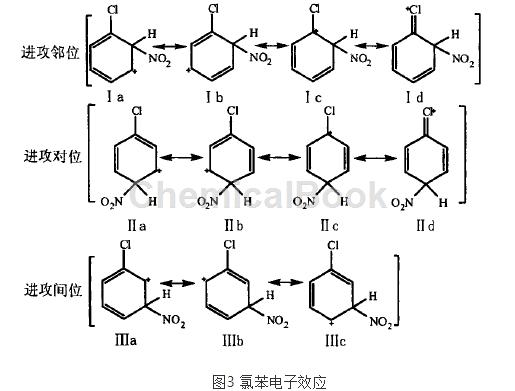
from the perspective of steric effect, the chlorine atom is larger in size and hinders the ortho position of n02+, thus reducing the formation of ortho products.
2 reaction process in microchannel
compared with the traditional batch reaction process, it has the advantages of rapid mixing, efficient heat transfer, narrow residence time distribution, good repeatability, rapid system response, easy automatic control, almost no amplification effect, and high safety performance. the nitration of chlorobenzene is a rapid and strong exothermic reaction. in the traditional trans reactor, there are shortcomings such as the heat released by the reaction cannot be released in time, the reaction temperature cannot be accurately controlled, and the reaction liquid is unevenly stirred, which can easily cause side reactions and process problems. complex operations, production safety and other issues. in microchannel reactors, size miniaturization strengthens the heat and mass transfer processes of the equipment and achieves process continuity. experiments were conducted to study the effects of main factors such as raw material ratio, reaction temperature, and volumetric flow rate on the conversion rate and selectivity of chlorobenzene.
2.1 selectivity comparison
in the experiment, under the respective optimal process conditions of the microchannel reactor and the conventional reactor, the comparative product selectivity results are shown in table 1.
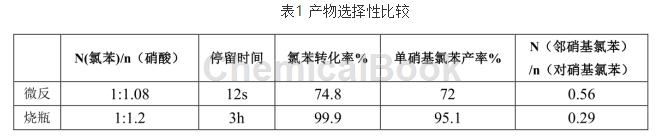
table 1 product selectivity comparison
the results in table 1 show that in the microchannel reactor, although the single-pass conversion rate of chlorobenzene is relatively low, the ortho-selectivity of the obtained product is significantly improved, and the by-products are relatively small. analyzing the reasons, the microchannel reactor with miniaturized size strengthens the heat transfer and mass transfer processes, weakens the ortho steric hindrance effect in the reaction, facilitates the generation of o-nitrochlorobenzene, and improves the ortho selectivity of chlorobenzene.
2.2 comparison of space-time conversion rates
the space-time conversion rate (stc) is defined as: stc=△n/(v.h)mol.m-3.h-1, which is the number of moles of reactants converted into products per unit volume and unit time. it is a measure of the reaction. one of the signs of device production capacity. in the experiment, under respective optimal process conditions, the space-time conversion rate of the chlorobenzene confirmation reaction in the microchannel reactor and the conventional reactor was compared, as shown in table 2.
table 2 comparison of spatiotemporal conversion rates
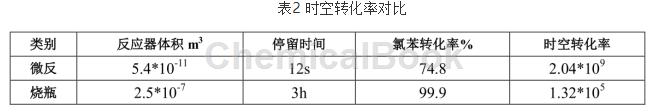
the results in table 2 show that the space-time yield in the microchannel reactor is relatively high, about 4 orders of magnitude higher than that in conventional reactors. when expanding productivity and production, it is only necessary to increase the number of microchannel reactors in series and parallel, respectively, which is the so-called “quantity amplification”.
references
[1]antes j, boskovic d, krause h, et al. analysis and improvement of strong exothermic nitrations in microreactors[j].chem.eng.res.des.,2003,81(7):760-765 .
[2]ducry l, roberge, d m. controlled autocatalytis nitration of phenol in a microreactor[j].angew.chem.int.ed.,2005,44(48),7972-7975.
[3]roberge d m,ducry l,bieler n,et al. microreactor technology: a revolution for the fine chemical and pharmaceutical industries[j].chem.eng.technol.,2005,28(3):318- 323.

 微信扫一扫打赏
微信扫一扫打赏

