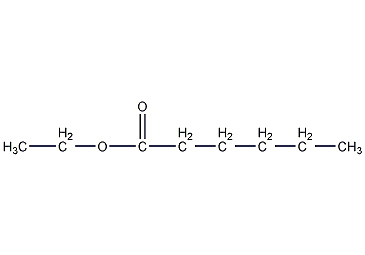
Structural formula
| Business number | 03GY |
|---|---|
| Molecular formula | C8H16O2 |
| Molecular weight | 144.21 |
| label |
Ethyl capronate, Ethyl n-hexoate, Caproic acid ethyl ester, food additives |
Numbering system
CAS number:123-66-0
MDL number:MFCD00009511
EINECS number:204-640-3
RTECS number:MO7735000
BRN number:1701293
PubChem ID:None
Physical property data
1. Properties: colorless to yellow liquid with pleasant odor. [1]
2. Melting point (℃): -67[2]
3. Boiling point (℃): 161.9~168[3]
4. Relative density (water=1): 0.869[4]
5. Relative vapor density (air = 1): 5.0[5]
6. Octanol/water partition coefficient: 2.83[6]
7. Flash point (℃): 54.4 (OC) [7]
8. Solubility: insoluble in water, glycerin, soluble in ethanol, ether, etc. Most organic solvents. [8]
Toxicological data
1. Skin/eye irritation: Rabbit skin standard Drez eye dye test: 500mg/24H has a moderate irritating effect on the skin.
2. It has low toxicity and no adverse effects on the human body. It has anesthetic properties at high concentrations. Irritating to respiratory tract, eyes and skin if inhaled.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[9] This substance May be harmful to the environment, special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 40.88
2. Molar volume (cm3/mol): 164.0
3. Isotonic specific volume (90.2K ): 375.1
4. Surface tension (dyne/cm): 27.3
5. Polarizability (10-24cm3): 16.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 89.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[10] Stable
2. Incompatible substances [11]Acids, alkalis, strong oxidants, strong reducing agents
3.Conditions to avoid contact[12] Heat
4. Polymerization hazard[13] No aggregation
Storage method
Storage Precautions[14] Store in a cool, ventilated warehouse. The temperature should not exceed 37°C. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with leakage emergency response equipment and suitable containment materials.
Synthesis method
Ethyl hexanoate naturally exists in pineapples and other fruits. It is industrially obtained by esterification of hexanoic acid and ethanol, followed by washing, neutralization and drying. ![]()
Purpose
1. It is used as a variety of blending spices, flavoring essences, fruit flavors, and also has great use in the flavoring of wine. Used as organic solvents and in organic synthesis.
2. Ethyl hexanoate is an edible flavoring that is allowed to be used according to my country’s hygienic standards for the use of food additives. It is often used to prepare fruity flavorings such as apples, pineapples, and bananas, and alcoholic flavors such as brandy and liquor. The dosage is based on normal production needs. Generally, it is 32mg/kg in chewing gum; 12mg/kg in candies and baked goods; and 7mg/kg in cold drinks.
3. It plays the role of top fragrance in floral and fruity daily flavors. It is often used to prepare food flavors such as bananas, pineapples, and apples, as well as flavors for brandy and liquor. The dosage in candies and baked goods is 12×10-6, and the dosage in chewing gum is 32×10-6.
4. Used in organic synthesis and preparation of artificial flavors. [15]

 微信扫一扫打赏
微信扫一扫打赏

