Overview[1]
5-Hydroxy-2-methoxy-benzaldehyde is a phenolic structure. The synthesis method of phenolic compounds includes the following steps: in the presence of a cuprous catalyst and ammonia water, an arylboronic acid compound and MOH are Substitution reaction yields phenolic compounds.
Preparation method[1-2]
Method 1,
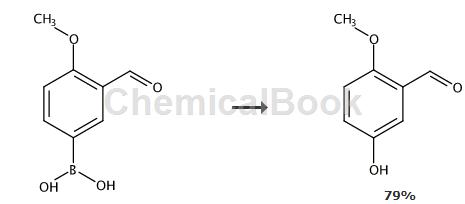
Preparation of 2-methoxy 5-hydroxybenzaldehyde
Add 0.0144g cuprous oxide (0.1mmol) and 0.192mL (2.5mmol) NH3-H2O into a round-bottomed flask equipped with a magnetic stirrer. ), 0.180g (1mmol) of 3-aldehyde-4-methoxyphenylboronic acid, 0.2g (5mmol) of sodium hydroxide, 2mL of water. At 15°C, open system, reaction time is 24 hours. After the reaction is completed, add hydrochloric acid to adjust the pH value to 2-3, and extract with ethyl acetate three times, 10 mL each time. The combined organic phase is concentrated, separated and purified to obtain 120 mg of 2-methoxy 5-hydroxybenzaldehyde. The rate is 79%.
Product 2-methoxy 5-hydroxybenzaldehyde: 1H NMR (CDCl3, 600MHz) δ10.4 (s, 1H), 7.36 (d , 1H, J=2.8Hz), 7.12 (dd, 1H, J=2.8, 8.9Hz), 6.91 (d, 1H, J=8.9Hz), 5.73 (s, 1H), 3.88 (s, 3H).13C NMR (CDCl3, 150MHz) δ190.1, 156.6, 149.8, 125.0, 123.6, 113.7, 113.4, 56.1.ESI-MS[M-H]– m/z 151.5.
Method 2:
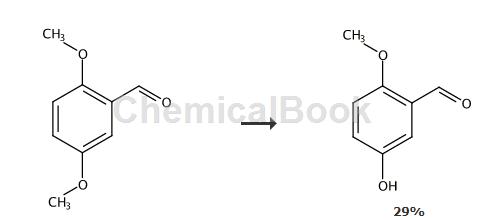
At 0°C, slowly add concentrated H2SO4 (40mL) to 2,5-dimethoxybenzaldehyde (3.32g, 20mmol) middle. The solution was heated at 55°C and stirred for 46 hours. The reaction was quenched with water and the product was extracted first with Et2O and then with aqueous NaOH. The solution was acidified with aqueous HCl and extracted with Et2O. The solution was dried with MgSO4 and concentrated by evaporation. The crude product was subjected to SiO2 column chromatography (CHCl3/MeOH), followed by precipitation from MeOH/H2O to obtain the product. Light brown solid, yield 0.9 g, 29%. 1H NMR (CDCl3): δ3.87 (s, 3H, -OCH3), 6.57 (s, 1H, -OH) , 6.89 (d, J = 9.2Hz, 1H), 7.13 (dd, Ja = 9.2Hz, Jb = 3.1Hz, 1H), 7.37 (d, J = 3.1Hz, 1H), 10.38 (s, 1H, -CHO ). 13CNMR (CDCl3, 125MHz): δ190.53,156.58,149.95, 124.82,123.96,113.71,113.31,56.14.
Main reference materials
[1] [Chinese invention] CN201110063336.9 A method for synthesizing phenolic compounds
[2] Suzuki Y , Hashimoto K , Tajima K . Synthesis of Regioregular Poly(/r, p/r, -phenylenevinylene)s by Horner Reaction and Their Regioregularity Characterization[J]. Macromolecules, 2007, 40(18) :6521-6528.

 微信扫一扫打赏
微信扫一扫打赏

