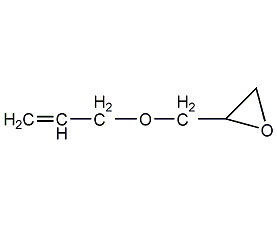
Structural formula
| Business number | 02U6 |
|---|---|
| Molecular formula | C6H10O2 |
| Molecular weight | 114.14 |
| label |
Allyl glycidyl ether, 1-allyloxy-2,3-epoxypropane, Age, lifespan, aging, Allyl-2,3-epoxypropyl ether, Epoxy resin reactive diluent XY680, Allylglycidyl ether, 1,2-Epoxy-3-allyloxypropane, 1-Allyloxy-2,3-epoxypropane, Allyl 2,3-epoxypropyl ether, Allyl glycidyl ether, Glycidyl allyl ether, ((2-Propenyloxy)methyl)-oxiran, ((2-Propenyloxy)methyl)oxirane, [(2-Propenyloxy)methyl]-oxiran, Aliphatic ether raw materials |
Numbering system
CAS number:106-92-3
MDL number:MFCD00005143
EINECS number:203-442-4
RTECS number:RR0875000
BRN number:105871
PubChem number:24845890
Physical property data
1. Properties: colorless liquid [1]
2. Melting point (℃): -100 [2]
3. Boiling point (℃): 154[3]
4. Flash point (℃): 57[4]
5. Density: 0.962[5]
6. Solubility: No data yet
Toxicological data
1. Acute toxicity: rat oral LD5O: 920mg/kg; rabbit skin LD5O: 2550mg/kg; rat inhalation LC5O: 860ppm/4H.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 30.89
2. Molar volume (cm3/mol): 116.3
3. Isotonic specific volume (90.2K ): 277.0
4. Surface tension (dyne/cm): 32.1
5. Polarizability (10-24cm3): 12.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 21.8
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 80.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
���Avoid contact with acids, alkalis, oxidants, and air.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
The preparation methods of allyl glycidyl ether can be divided into two main types. The first method is to use allyl alcohol and epichlorohydrin as raw materials, and perform ring-opening addition under the action of Lewis acid (H2SO4, BF3·Et2O, HClO4, etc.) An intermediate is obtained, and then the intermediate is removed from the HCl ring under the action of an alkali solution (NaOH solution, etc.) to obtain the product. The reaction formula is as follows: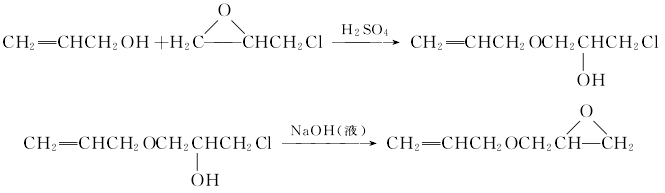
The second method is phase transfer catalysis. It uses allyl alcohol and epichlorohydrin as raw materials, and reacts under the action of sodium hydroxide solution and phase transfer catalyst to directly generate allyl glycidyl ether. The reaction formula is as follows: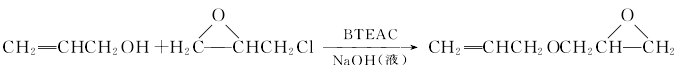
Purpose
1. Used to synthesize coupling agents, and can also be used as fiber modifiers, stabilizers for chlorinated organic compounds, reactive diluents for synthetic resins, and reformers. As an allylic or epoxy function with differential reactivity, used in elastomers, epoxy resins, adhesives and fibers, coating reactive intermediates, glass fiber surface repair agents; scale inhibitors, as unsaturated polyesters Air-drying agent, it can also be used as an electronic coating silicone intermediate. [6]

 微信扫一扫打赏
微信扫一扫打赏

