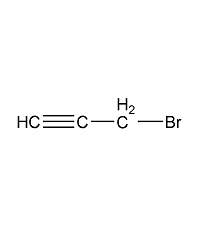
Structural formula
| Business number | 02UA |
|---|---|
| Molecular formula | C3H3Br |
| Molecular weight | 118 |
| label |
Aliphatic halogenated derivatives |
Numbering system
CAS number:106-96-7
MDL number:MFCD00000241
EINECS number:203-447-1
RTECS number:UK4375000
BRN number:605309
PubChem ID:None
Physical property data
1. Properties: Colorless to bright yellow liquid with a special pungent odor.
2. Density (g/mL, 25℃): 1.52
3. Relative vapor density (g/mL, air=1): 6.87
4. Melting point (ºC): -61.1
5. Boiling point (ºC, normal pressure): 82.5
6. Relative density (20℃, 4℃): 1.571
7. Refractive index (nD20): 1.4929
8. Flash point (ºC): 18
9. Specific rotation (ºC): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC) : Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14 . Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19 . Solubility: Soluble in ethanol, ether, and benzene.
Toxicological data
1. Acute toxicity: Rat oral LD50: 53mg/kg; Guinea pig oral LD50: 29μg/kg;
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 21.50
2. Molar volume (cm3/mol): 75.0
3. Isotonic specific volume (90.2K ): 182.6
4. Surface tension (dyne/cm): 35.1
5. Polarizability (10-24cm3): 8.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 38.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants and strong alkali.
2 It is toxic if swallowed and inhaled. The steam is highly irritating and can cause harm to the eyes, nose and skin. This product is a strong tear-triggering agent.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials. 2. The outer wooden box or calcium plastic box of the glass bottle is reinforced with padding. Store in a cool, dry and ventilated warehouse. Keep away from heat, fire and direct sunlight. Store and transport separately from oxidants and other conflicting materials.
Synthesis method
1. Obtained from the reaction of propargyl alcohol and phosphorus bromide. Add propargyl alcohol to pyridine under cooling, stir and slowly add phosphorus tribromide dropwise at -5°C, and maintain the reaction temperature below 0°C. After the addition is complete, continue stirring for 15 minutes. Then, distill under reduced pressure, collect all the distillate, and fractionate again under normal pressure to obtain the finished product. The preparation method is to add hydrogen bromide and solvent into the reaction bottle, add propargyl alcohol dropwise under stirring in the presence of CuBr and Cu catalyst, slightly heat up the reaction after the dripping is completed, and then post-process and distillate to obtain bromopropyne. . ![]() Or use propargyl alcohol and (PhO)3PBr 2The product can also be obtained by reacting in the presence of pyridine.
Or use propargyl alcohol and (PhO)3PBr 2The product can also be obtained by reacting in the presence of pyridine.
Or add a small amount of pyridine to dry propargyl alcohol, add phosphorus tribromide and a small amount of pyridine solution dropwise at 0°C while stirring, stir for 20 minutes after the drops are completed, and distill under reduced pressure to obtain the product. 2. Preparation method: ![]()
Equipped with stirrer, thermometer, reflux condenser, dripper In the reaction bottle of the liquid funnel, add 112g (2.0mol) of propargyl alcohol (2), cool to below 0℃ in an ice-salt bath while stirring, slowly add 18g of pyridine ①, and cool to -5℃ Next, add 200g (0.74mol) of phosphorus tribromide dropwise, control the dropping temperature not to exceed 0°C, complete the addition in about 1.5h, and then continue to stir the reaction for 0.5h. Distill under reduced pressure with a water pump, collect all the distillates, and then fractionate at normal pressure to collect the fractions at 83-89°C to obtain 180g of propargyl bromide (1), with a yield of 76%. Note: ① Heat is released during the addition process. Be careful not to add it all at once to avoid temperature rise. [1]
Purpose
Used to synthesize various derivatives of acetylene; used in the pharmaceutical industry to manufacture the antifungal drug chloropropyne iodine.

 微信扫一扫打赏
微信扫一扫打赏

