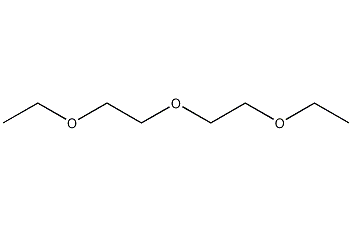
Structural formula
| Business number | 035J |
|---|---|
| Molecular formula | C8H18O3 |
| Molecular weight | 162.23 |
| label |
Bis(2-ethoxyethyl) ether, 2-ethoxyethyl ether, Diethyl carbitol, Diethylene glycol diethyl ether, 2-ethoxyethyl ether, 2-Ethoxyethyl ether, Bis(2-ethoxyethyl) ether, Diethyl carbitol, Uranium ore extraction agent, Leveling agent for fiber and leather, Leveling agent for photographic printing, High boiling point reaction medium |
Numbering system
CAS number:112-36-7
MDL number:MFCD00009254
EINECS number:203-963-7
RTECS number:KN3160000
BRN number:1699259
PubChem number:24859284
Physical property data
1. Properties: colorless liquid
2. Relative density (g/mL, 20/20℃): 0.9082
3. Relative density (20℃, 4℃ ): 0.9063
4. Freezing point (ºC): -44.3
5. Boiling point (ºC, normal pressure): 189
6. Refractive index (20ºC ): 1.4115
7. Viscosity (mPa·s, 20ºC): 1.40
8. Flash point (ºC, opening): 82
9. Relative Density (25℃, 4℃): 0.886342
10. Vapor pressure (kPa, 20ºC): 0.05
11. Vapor pressure (kPa, 72ºC): 1.33
12. Vapor pressure (kPa, 107ºC): 6.67
13. Heat of evaporation (KJ/mol): 48.99
14. Normal temperature Refractive index (n20): 1.4115
15. Critical temperature (ºC): 338.85
16. Specific heat capacity (KJ/(kg·K), Constant pressure): 2.11
17. Volume expansion coefficient (K-1): 0.00106
18. van der Waals volume (cm3 ·mol-1): 99.820
19. Solubility: Miscible with alcohol and other organic solvents, completely miscible with water at 20°C. Can dissolve rosin, glycerol trirosinate, grease, ethyl cellulose, nitrocellulose, chloroprene rubber, polystyrene and alkyd resin, etc.
20. Solubility parameter (J·cm-3)0.5: 17.651
21. van der Waals area ( cm2·mol-1): 1.414×1010
22. Liquid phase standard hot melt (J· mol-1·K-1): 340.3
Toxicological data
1. Acute toxicity: guinea pig oral LD50: 1850 mg/kg; rat oral LD50: 4790 mg/kg; rabbit dermal LD50: 6700 mg/kg
Ecological data
Slightly harmful to water bodies.
Molecular structure data
1. Molar refractive index: 44.34
2. Molar volume (cm3/mol): 179.6
3. Isotonic specific volume (90.2K ): 409.4
4. Surface tension (dyne/cm): 26.9
5. Polarizability (10-24cm3): 17.58
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 58.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxides and acids. Peroxide will be generated during storage and needs to be removed with a reducing agent during distillation. Calcium chloride and magnesium sulfate can be used as desiccants, and metallic sodium, calcium hydride or lithium aluminum hydride can be used for further drying.
2. It has the chemical properties of ether.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep away from fire sources to prevent explosion. Store away from oxidizing and acidic substances.
Synthesis method
Obtained from the reaction of diethylene glycol and ethanol. Another preparation method is to react diethylene glycol monoethyl ether with ethyl bromide in the presence of metallic sodium (or sodium hydroxide).
Refining method: remove peroxide and then distill under reduced pressure.
Purpose
It is used as an oil-water mixed solvent for printing and dyeing nitrocellulose and wool fabrics and as an extraction agent for uranium ore. It is also a reaction medium with a high boiling point. It is used as an organic synthetic solvent, a component of nitrocellulose spray paint for brushing, a leveling agent for fibers and leather, a leveling agent for photographic printing, etc.

 微信扫一扫打赏
微信扫一扫打赏

