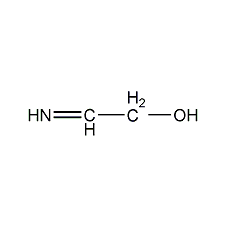
Structural formula
| Business number | 02UV |
|---|---|
| Molecular formula | C3H6O |
| Molecular weight | 58.08 |
| label |
2-propen-1-ol, Propylene-[2]-alcohol-[1], allyl alcohol, 1-propen-3-ol, propylene alcohol, Alliol, ethylene methanol, 2-propenol, 2-Propylene-1-ol, Allyl alcohol, 1-Propylene-3-ol, Propylene alcohol, Vinyl carbinol, Aliphatic alcohols, ethers and their derivatives |
Numbering system
CAS number:107-18-6
MDL number:MFCD00002920
EINECS number:203-470-7
RTECS number:BA5075000
BRN number:605307
PubChem number:24854419
Physical property data
1. Properties: Colorless liquid with pungent tear-jerking mustard-like odor, irritating eyes.
2. Boiling point (ºC, 101.3kPa): 95-97
3. Melting point (ºC): -50
4. Relative density (g/ mL, 20/4ºC): 0.8524
5. Relative density (g/mL, 25/4ºC): 0.8476
6. Relative vapor density (g/mL, air=1 ): 2.00
7. Refractive index (n20ºC): 1.4135
8. Refractive index (n25ºC): 1.4111
9. Refractive index (30ºC): 1.4029
10. Viscosity (mPa·s, 15ºC): 1.486
11. Viscosity (mPa·s, 20ºC): 1.361
12. Viscosity ( mPa·s, 30ºC): 1.072
13. Flash point (ºC, closed cup): 22
14. Flash point (ºC, open cup): 32
15. Ignition point (ºC): 378
16. Heat of evaporation (KJ/kg): 39.99
17. Heat of combustion (KJ/kg): 1853.9
18. Specific heat capacity (KJ/(kg·K), 20~95.5ºC, liquid): 2.78
19. Relative evaporation rate (ether=1): 11
20. Critical temperature (ºC): 271.9
21. Thermal conductivity (W/(m·K), 20ºC): 0.1754
22. Vapor pressure (kPa, 20ºC ): 2.31
23. Lower explosion limit (%, V/V): 2.50
24. Upper explosion limit (%, V/V): 18.0
25. Solubility: can be dissolved in water, ethanol, chloroform, ether and many other
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 25°C in hot seasons. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, alkali metals, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Packed in tin drums. Store and transport according to regulations for flammable and toxic substances.
Synthesis method
Its preparation methods include the following.
Isomerization of propylene oxide
After gasification and preheating, propylene oxide enters the suspended bed reactor through the distributor. At (200±5)℃, 98~196kPa pressure, aluminum phosphate is used as the catalyst , the finished product is obtained from the isomerization of propylene oxide.
Acrolein reduction method
Using acrolein as the raw material, isopropyl alcohol as the reducing agent, the reaction temperature is 400°C, and magnesium oxide and zinc oxide are used as catalysts to obtain the finished product.
Prepare propylene alcohol from propylene
Use propylene as raw material, react with acetic acid and oxygen over a precious metal catalyst to obtain propylene acetate. The reaction is carried out in a fixed bed. The obtained propylene acetate is heated at 170~250℃ and Hydrolysis under a pressure of at least 500 kPa can produce propylene alcohol and acetic acid. 1. Propylene oxide isomerization method. This process is simple, has high yield, and is corrosion-free, so it is the most widely used. This method is divided into two types: gas phase method and liquid phase method. my country adopts gas phase method. The description is as follows: After gasification and preheating, propylene oxide enters the suspended bed reactor through a distributor and is isomerized to obtain allyl alcohol at 280°C ± 5°C and a pressure of 11.96MPa in the presence of a lithium phosphate catalyst. 2. Alkaline hydrolysis of allyl chloride: It was researched and developed by the American Shell Oil Company and Dow Chemical Company in 1947 and is still the main industrial production method used today. 3. Acrolein reduction method: In the late 1950s, propylene was used as raw material and was oxidized to acrolein; then it was hydrogenated with isopropanol to obtain propenol and acetone. 4. Propylene acetate method The development of propylene acetate technology has provided an effective way for the large-scale industrial production of propylene alcohol and the development of its derivatives. Simple process flow: (1) Propylene chloride hydrolysis method Propylene chloride is hydrolyzed in 5%-10% NaOH aqueous solution at 150°C, 1.3-1.4MPa and a pH value of 10-12 to obtain propenol, yield It is about 85%-95%, and by-products include 5%-10% diallyl ether, propionaldehyde and high boiling substances.  (2) Acrolein reduction method is an intermediate step in the acrolein method to synthesize glycerol. Under the action of a catalyst, propylene is first oxidized to acrolein, and then acrolein is hydrogen-exchanged with ethanol or isopropyl alcohol to obtain propenol. For example, under the conditions of 400℃, 0.1MPa, MgO and ZnO as catalysts, acrolein reacts with isopropanol and generates propenol and acetone through hydrogen transfer.
(2) Acrolein reduction method is an intermediate step in the acrolein method to synthesize glycerol. Under the action of a catalyst, propylene is first oxidized to acrolein, and then acrolein is hydrogen-exchanged with ethanol or isopropyl alcohol to obtain propenol. For example, under the conditions of 400℃, 0.1MPa, MgO and ZnO as catalysts, acrolein reacts with isopropanol and generates propenol and acetone through hydrogen transfer. 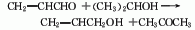 (3) Propylene oxide isomerization method By using propylene oxide under the action of lithium phosphate catalyst, the liquid Phase or gas phase isomerization gives allyl alcohol. First, propylene oxide is vaporized and preheated, and then enters the suspended bed reactor through the distributor. The catalyst is suspended in the solvent. The lithium phosphate content is 15%-25% (mass). At 275-285℃, 0.1-0.2MPa, the liquid air The suspension reaction is carried out at a speed of 1.7-2.5L liquid propylene oxide/(kg catalyst·h). This method is currently the main method for producing propylene alcohol. The gas phase method uses a fixed reactor, propylene oxide is catalyzed by lithium phosphate, and the reaction temperature is 250-350°C. The gas phase method is better than the liquid phase method. The by-products are acetone and propionaldehyde.
(3) Propylene oxide isomerization method By using propylene oxide under the action of lithium phosphate catalyst, the liquid Phase or gas phase isomerization gives allyl alcohol. First, propylene oxide is vaporized and preheated, and then enters the suspended bed reactor through the distributor. The catalyst is suspended in the solvent. The lithium phosphate content is 15%-25% (mass). At 275-285℃, 0.1-0.2MPa, the liquid air The suspension reaction is carried out at a speed of 1.7-2.5L liquid propylene oxide/(kg catalyst·h). This method is currently the main method for producing propylene alcohol. The gas phase method uses a fixed reactor, propylene oxide is catalyzed by lithium phosphate, and the reaction temperature is 250-350°C. The gas phase method is better than the liquid phase method. The by-products are acetone and propionaldehyde. ![]() (4) Propylene acetate hydrolysis method Propylene is acetoxylated to produce propylene acetate, which is then Hydrolysis or transesterification produces propylene alcohol. A new type of catalyst that uses palladium as the catalyst, potassium acetate or acetic acid-copper acetate as the cocatalyst, silica as the carrier, and organic bases such as ethylenediamine as the accelerator, selectively performs an oxyacetylation reaction at the propylene position. . The reaction temperature is 160-180°C, the reaction pressure is 0.5-1MPa, and the selectivity of propylene acetate is about 94%. The reaction conditions are mild, production can be carried out stably, and the production of a large number of by-products is avoided.
(4) Propylene acetate hydrolysis method Propylene is acetoxylated to produce propylene acetate, which is then Hydrolysis or transesterification produces propylene alcohol. A new type of catalyst that uses palladium as the catalyst, potassium acetate or acetic acid-copper acetate as the cocatalyst, silica as the carrier, and organic bases such as ethylenediamine as the accelerator, selectively performs an oxyacetylation reaction at the propylene position. . The reaction temperature is 160-180°C, the reaction pressure is 0.5-1MPa, and the selectivity of propylene acetate is about 94%. The reaction conditions are mild, production can be carried out stably, and the production of a large number of by-products is avoided. ![]()
Purpose
1. Used for the preparation of propylene compounds, synthesis of resins and plastics, and analytically used for microscopic analysis and determination of mercury, etc. Polymerization monomers are widely used as plasticizers, cross-linking agents, etc., and are also used as manufacturing monomers for lens materials and coatings. It is an important intermediate in the manufacture of glycerin, glycidol, pharmaceuticals, fragrances, cosmetics and agricultural chemicals, and is also used in the coating and glass fiber industries.
2.Polymerization monomer is also an important intermediate for the synthesis of medicines, pesticides and spices. Used in the manufacture of diallyl phthalate, glycerol, allyl glycerol, epichlorohydrin, allyl acetate, allyl butyrate, allyl caproate, allyl acrylate, trimellitamine Allyl formate, allyl acetoacetate, allyl thiourea, etc. It is widely used as plasticizer, cross-linking agent, flame retardant, antioxidant, surfactant, fiber treatment agent, etc. It is also used as a manufacturing monomer for lens materials and coatings.
3. Used as a fixative in microscope analysis, acrylic compound preparation, resin, plastic synthesis, etc.
Activators, fiber treatment agents, etc. are also used as manufacturing monomers for lens materials and coatings.
3. Used as a fixative in microscope analysis, acrylic compound preparation, resin, plastic synthesis, etc.

 微信扫一扫打赏
微信扫一扫打赏

