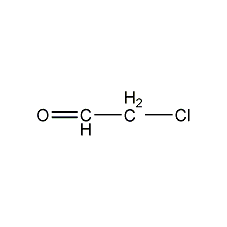
Structural formula
| Business number | 02UX |
|---|---|
| Molecular formula | C2H3ClO |
| Molecular weight | 78 |
| label |
Pesticide intermediates; aliphatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:107-20-0
MDL number:MFCD00006992
EINECS number:203-472-8
RTECS number:AB2450000
BRN number:1071226
PubChem number:24853642
Physical property data
1. Properties: 40% aqueous solution is a colorless and transparent oily liquid with a pungent odor. [1]
2. Melting point (℃): -16.3 (40%) [2]
3. Boiling point (℃): 85~86 (40%)[3]
4. Relative density (water=1): 1.19 (40%)[4]
5. Relative vapor density (air=1): 2.7[5]
6. Saturated vapor pressure (kPa): 13.3 ( 45℃, 40%)[6]
7. Critical pressure (MPa): 5.37[7]
8 .Octanol/water partition coefficient: 0.09[8]
9. Flash point (℃): 87.8 (CC)[9]
10. Ignition temperature (℃): 88[10]
11. Solubility: soluble in water, soluble in ethanol, ether, chloroform and most other Organic solvents. [11]
Toxicological data
1. Acute toxicity[12] LD50: 50~400mg/kg (rat oral)
2. Irritation No data available
3. Subacute and chronic toxicity[13] Rats were injected with doses of 0.3 and 0.6 LD50, and 25% and 67% of the animals died respectively after 30 days. The animals developed significant bronchitis and pneumonia.
4. Mutagenicity[14] Microbial mutagenicity: Salmonella typhimurium 400 μmol/L; Escherichia coli 1mmol/L. DNA damage: human lymphocytes 100 μmol/L. Mammalian somatic mutations: hamster lung 6400nmol/L.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradable[15] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 1.7d (theoretical).
Molecular structure data
1. Molar refractive index: 16.35
2. Molar volume (cm3/mol): 70.2
3. Isotonic specific volume (90.2K ): 159.0
4. Surface tension (dyne/cm): 26.2
5. Polarizability (10-24cm3): 6.48
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 20
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. NoDetermine the number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible materials[17] Strong oxidizing agent
3. Polymerization Hazard[18] Does not polymerize
4. Decomposition products[19] Hydrogen chloride
Storage method
Storage Precautions[20] Store in a cool, well-ventilated dedicated warehouse. Implement the system of “dual people to send and receive, and double to keep”. Keep away from fire and heat sources. The storage temperature does not exceed 32°C and the relative humidity does not exceed 80%. Keep container tightly sealed. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained by chlorination of vinyl chloride in water. First add water to the chlorination reaction tower, introduce chlorine and vinyl chloride at 40-45°C and react for 6-7 hours. The reaction ends when the chloroacetaldehyde content reaches 9-10%. The by-product trichloroethane is separated and the finished product is obtained. Commercial chloroacetaldehyde is a 30%, 40% or 50% aqueous solution.
![]()
Purpose
1. Used in organic synthesis, such as the preparation of sulfathiazole, mercaptoacetaldehyde and fungicides. Also used to make tree trunks easier to peel.
2. Used in organic synthesis and as a fungicide. [21]

 微信扫一扫打赏
微信扫一扫打赏

