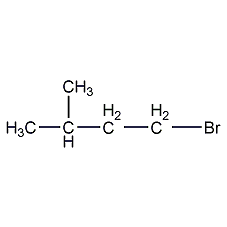
Structural formula
| Business number | 02VS |
|---|---|
| Molecular formula | C5H11Br |
| Molecular weight | 151.04 |
| label |
1-Bromo-3-methylbutane, 1-bromoisopentane, Bromoisopentane, Isoamyl bromide, Isoamyl bromide, Isopentyl bromide, Aliphatic halogenated derivatives |
Numbering system
CAS number:107-82-4
MDL number:MFCD00000253
EINECS number:203-522-9
RTECS number:EJ6230000
BRN number:773652
PubChem number:24847596
Physical property data
1. Properties: colorless to light yellow liquid.
2. Density (g/mL, 25℃): 1.2090
3. Relative density (20℃, 4℃): 1.2071
4. Melting point (ºC): -112
5. Boiling point (ºC, normal pressure): 120.5
6. Relative density (25℃, 4℃): 1.2006
7. Refraction Rate (D20): 1.4420
8. Flash point (ºC): 21
9. Refractive index at room temperature ( n25): 1.4400
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, soluble in alcohol, ether and chloroform.
Toxicological data
1. Acute toxicity: rat intraperitoneal LD50: 6150mg/kg; mouse intraperitoneal LD50: 420mg/kg; mammal inhalation LC50: 21300mg/m3;
Ecological data
This substance is harmful to the environment and can cause pollution to the atmosphere.
Molecular structure data
1. Molar refractive index: 32.90
2. Molar volume (cm3/mol): 124.6
3. Isotonic specific volume (90.2K ): 280.6
4. Surface tension (dyne/cm): 25.6
5. Polarizability (10-24cm3): 13.04
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptorsAmount: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 25.1
10. Isotopes Number of atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine chemical bonds Number of stereocenters: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants and strong alkali.
2. See 1,4-dibromopentane. When the concentration is high, it will damage the liver and have an anesthetic effect. The human body inhales LC5021300mg/m3 and intraperitoneally injects LD50480mg/kg.
This product is produced for self-use, see 1,4-dibromopentane.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The storage temperature should not exceed 30℃. Keep container tightly sealed. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of isoamyl alcohol and hydrobromic acid in the presence of sulfuric acid. Add amyl alcohol and hydrobromic acid to the reaction tank according to the ingredients ratio, stir and heat to 95-100°C, and reflux for 1 hour. At this temperature, slowly add sulfuric acid, and react at 100-106°C for 1.5 hours until the hydrobromic acid does not overflow. Distill and collect the distillate in a separator. After washing with water, add 5-7% sodium carbonate solution to adjust pH=8-9, separate the water layer, and then wash with water until pH=5-6 of the washing liquid. Separate the washing liquid, add calcium chloride for dehydration, and distill under reduced pressure. Collect the 118-122°C (21.3-14.7kPa) fractions to obtain the product. The yield is 78% based on isoamyl alcohol. The purity of industrial product isopentyl is above 98%. Raw material consumption quota: isoamyl alcohol 792kg/t; bromine 900kg/t; sulfuric acid 750kg/t.
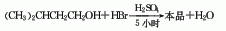
2. Preparation method:
p>
![]()
Use 210g (48%) hydrogen bromide Acid, 60g concentrated sulfuric acid, 88g (1.0mol) 3-methyl-1-butanol (2), and 10g concentrated sulfuric acid, refer to the preparation method of 1-bromopropane, use a fractionating column to evaporate the product, collect 117~120 ℃ fraction, 125g of 1-bromo-3-methylbutane (1) was obtained, with a yield of 83%. [1]
Purpose
Solvents; pesticide raw materials; preparation of pesticides and fungicides; fire extinguishing agents and flame retardant raw materials; refrigerant raw materials; mineral processing agent raw materials; pharmaceutical raw materials; organic synthesis intermediates.

 微信扫一扫打赏
微信扫一扫打赏

