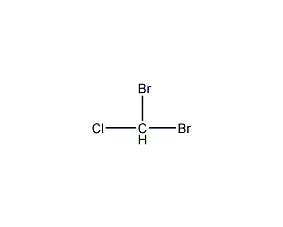
Structural formula
| Business number | 03JA |
|---|---|
| Molecular formula | CHBr2Cl |
| Molecular weight | 208.28 |
| label |
Monochlorodibromomethane, Chlorodibromomethane, Dibromochloromethane, Chlorodibromomethane, Dibromochloromethane, Chlorodibromomethane, Halogenated hydrocarbon solvents, aliphatic compounds |
Numbering system
CAS number:124-48-1
MDL number:MFCD00000820
EINECS number:204-704-0
RTECS number:PA6360000
BRN number:1731046
PubChem number:24863012
Physical property data
1. Properties: colorless liquid.
2. Boiling point (ºC, 101.3kPa): 121.3~121.8
3. Melting point (ºC): -22
4. Relative density (g/ mL, 15/4ºC): 2.4450
5. Refractive index (20ºC): 1.5471
6. Solubility: miscible with ethanol, ether, and benzene in any proportion.
7. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -9.0
8. Gas phase standard entropy (J·mol-1·K-1): 327.90
9. Gas phase standard free energy of formation (kJ·mol-1) : -6.9
10. Gas phase standard hot melt (J·mol-1·K-1): 69.17
Toxicological data
Toxic if taken orally. There is the possibility of irreversible damage to the body. The oral LD50 of male and female rats is 370mg/kg and 760mg/kg.
Ecological data
None
Molecular structure data
1. Molar refractive index: 26.95
2. Molar volume (cm3/mol): 83.1
3. Isotonic specific volume (90.2K ): 212.0
4. Surface tension (dyne/cm): 42.2
5. Dielectric constant:
6. Dipole moment (10-24cm3 ):
7. Polarizability: 10.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 13.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. UnsureNumber of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Dibromochloromethane is slightly more toxic than chloroform and has the common characteristics of halogenated hydrocarbon compounds, that is, it is highly irritating to the skin and mucous membranes. Protective clothing and gloves should be worn when using.
Storage method
Store at low temperature and protected from light.
Synthesis method
Brief description of production method: drinking water disinfection product.
Purpose
Make standard solutions and calibrate instruments and devices. Solvents, organic synthesis intermediates.

 微信扫一扫打赏
微信扫一扫打赏

