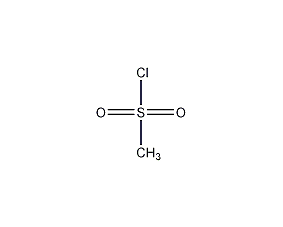
Structural formula
| Business number | 03JC |
|---|---|
| Molecular formula | CH3ClO2S |
| Molecular weight | 114.56 |
| label |
Methyl sulfonyl chloride, Methanesulfonyl chloride, Methanesulfonyl chloride, Sulfurylmethane chloride, Mesyl chloride, Aliphatic sulfur compounds |
Numbering system
CAS number:124-63-0
MDL number:MFCD00007454
EINECS number:204-706-1
RTECS number:PB2790000
BRN number:506297
PubChem ID:None
Physical property data
1. Properties: colorless or slightly yellow liquid[1]
2. Melting point (℃): -32[2]
3. Boiling point (℃): 164[3]
4. Relative density (water=1): 1.48[4]
5. Relative vapor density (air=1): 3.9[5]
6. Saturated vapor pressure (kPa): 1.60 (53 ℃)[6]
7. Critical pressure (MPa): 5.23[7]
8. Octanol/ Water partition coefficient: 1.27[8]
9. Flash point (℃): 110[9]
10. Solubility: Insoluble in water, soluble in ethanol, ether and most organic solvents. [10]
Toxicological data
1. Skin/eye irritation data Rabbit skin contact: severe reaction at 500mg; rabbit eye contact: serious reaction at 100mg;
2. Acute toxicity Rat oral LD50: 50 mg/kg; Rat Inhalation LCLo: 620 mg/m3/6H; rat intraperitoneal LDLo: 5mg/kg; mouse oral LD50: 200 mg/kg; mouse intraperitoneal LD50: 10 mg/kg; rodent-guinea pig skin contact LD50: 100 uL /kg;
3. Other multi-dose data Rat oral TDLo: 500 mg/kg/2W-I;
4. Mutagenicity data Bacteria – Salmonella typhimurium: 250 ug/plate; rodent-hamster ovary: 32 umol/L;
5. Acute toxicity[11] LD50: 50mg/kg (orally in rats); 200mg/kg (orally in mice)
6. Irritation[12] Rabbit eye: 5mg (30s), mild irritation.
7. Mutagenicity[13] Microbial mutagenicity: Salmonella typhimurium 250μg/dish. Cytogenetic analysis: hamster ovary 32μmol/L.
Ecological data
1. Ecotoxicity[14] LC50: 11mg/L (96h) (bluegill sunfish)
2. Biodegradability No data yet
3. Non-biodegradability[15] In the air, when the hydroxyl radical concentration is 5.00×105/cm3, the degradation half-life is 13d (theoretical).
Molecular structure data
1. Molar refractive index: 20.31
2. Molar volume (cm3/mol): 77.4
3. Isotonic specific volume (90.2K )��193.5
4. Surface tension (dyne/cm): 38.9
5. Polarizability (10-24cm3): 8.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 42.5
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 95.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Combustible when exposed to open flames or high heat. Can react with strong oxidizing agents. Decomposed by high heat to produce toxic corrosive gases. Corrosive.
2. Stability[16] Stable
3. Incompatible substances[17] Water, alcohols, strong oxidants, strong alkali
4. Conditions to avoid contact [18] Heat, Moist air
5. Polymerization hazard[19] Does not polymerize
6. Decomposition products[ 20] Hydrogen chloride, silicon oxide
Storage method
Storage Precautions[21] Store in a cool, dry and well-ventilated special warehouse, and implement “two people to send and receive, and two people to keep” “system. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, alcohols, etc. and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Brief description of production methods: There are several production methods as follows. 1. It is prepared by methylation of thiourea and oxidation of chlorine; 2. It is prepared by the reaction of methane sulfonic acid and thionyl chloride; 3. It is prepared by wet chlorination reaction using methyl mercaptan as raw material.
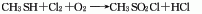
2. In 1 liter three neck Equip the bottle with a mechanical stirrer, reflux condenser, thermometer and separatory funnel, and place it in a fume hood. The bottle is filled with 152 grams (105 ml, 1.5 mol) of methanesulfonic acid. Heat to 95°C on a steam bath, and add 238 grams (146 ml, 2.0 mol) of thionite chloride dropwise within 4 hours. Maintain 95°C during the entire feeding process, and the reaction will be completed after 3.5 hours. Transfer the product into a distillation flask and install a Kirschner distillation head with a fractionating column. Heat in an oil bath (the bath temperature at the end of distillation does not exceed 115°C) and perform distillation under reduced pressure. First, evaporate thionyl chloride in Baowen.
![]()
Purpose
1. Methanesulfonyl chloride reacts easily with compounds with active hydrogen and can be used to react with amino groups and hydroxyl groups to introduce methane sulfonyl groups. This product can be used as raw material for the production of methanesulfonic acid, as a catalyst for esterification and polymerization reactions, as a liquid sulfur dioxide stabilizer, as a chlorinating agent for anthraquinone and carbazole reduction dyes, as a quick-drying agent for dry oil inks and coatings, and as a quick-drying agent for dry oil inks and coatings. Ester dyeing improver, color photo color regulator, xylene, naphthalene and formaldehyde condensation agent, wool dyeing auxiliary, etc. It is also widely used as a raw material for medicine and pesticides.
2. It is the raw material for producing methanesulfonic acid. The prepared methylsulfonamide is an important fine chemical product intermediate and can be widely used as a synthetic raw material for medicines and pesticides.
3. Used as analytical reagent. [22]

 微信扫一扫打赏
微信扫一扫打赏

