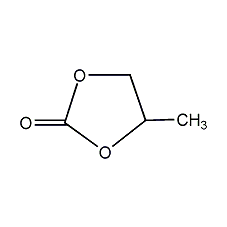
Structural formula
| Business number | 02WW |
|---|---|
| Molecular formula | C4H6O3 |
| Molecular weight | 102.09 |
| label |
1,2-Propanediyl carbonate, 1-Methylethylene carbonate, dispersants for plastics, Extracting agent, plasticizer, electrochemical solution, wood adhesive |
Numbering system
CAS number:108-32-7
MDL number:MFCD00005385
EINECS number:203-572-1
RTECS number:FF9650000
BRN number:107913
PubChem number:24887955
Physical property data
1. Properties: Colorless liquid, odorless, non-hygroscopic.
2. Density (g/mL, 20/20℃): 1.189
3. Relative density (20℃, 4℃): 1.2047
4 . Melting point (ºC): -50
5. Boiling point (ºC, normal pressure): 240
6. Relative density (25℃, 4℃): 1.198
7. Refractive index (20ºC): 1.4218
8. Flash point (ºC): 123
9. Viscosity (mPa·s, 40ºC): 1.38
10. Viscosity (mPa·s, 60ºC): 1.00
11. Refractive index at room temperature (n25): 1.4197
12. Saturated vapor pressure (kPa, 20ºC): 0.004
13. Heat of evaporation (KJ/mol, 150ºC): 55.27
14. Gas phase standard combustion heat (enthalpy) (kJ·mol -1): -1849.1
15. Conductivity (S/m): (1~2)×10-8
16. Specific heat capacity (KJ/(kg·K), 50ºC, constant pressure): 1.80
17. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -582.5
18. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -1818.4
19. Solubility : Soluble in water, alcohol, etherbenzene, carbon tetrachloride, ethyl acetate, chloroform, acetone and other solvents. Can selectively dissolve carbon dioxide from gas mixtures.
20. The liquid phase standard claims heat (enthalpy) (kJ·mol-1): -613.2
Toxicological data
1. Acute toxicity: Rat oral LD50: 29000mg/kg.
2. Animal tests have proven that there is no toxicity when taken or absorbed through the skin. Moderately irritating to the mucous membranes of the eyes and respiratory system, but not dangerous. Rats inhaled concentrated vapor for 8 hours without death.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 21.88
2. Molar volume (cm3/mol): 87.2
3. Isotonic specific volume (90.2K )��204.3
4. Surface tension (dyne/cm): 30.0
5. Dielectric constant:
6. Dipole moment (10 -24cm3):
7. Polarizability: 8.67
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 35.5
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 88.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants.
Chemical properties: Partial decomposition occurs above 200°C, and trace amounts of acid or alkali can promote decomposition. Propylene glycol carbonate can also hydrolyze rapidly at room temperature in the presence of acid, especially alkali.
2.The toxicity of this product is unknown. Pay attention to prevent phosgene poisoning during production. The workshop should be well ventilated and the equipment should be sealed. Operators should wear protective equipment.
3. Exist in flue-cured tobacco leaves and smoke.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
This product is packed in iron drums and stored in a cool and ventilated place, away from fire sources. Store and transport according to regulations on flammable chemicals.
Synthesis method
1. The raw material propylene glycol of the phosgene method reacts with phosgene to generate hydroxyisopropyl chloroformate, and then reacts with sodium hydroxide to generate propylene carbonate, which is then distilled under reduced pressure to obtain the finished product.
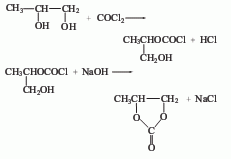
2. Transesterification method.
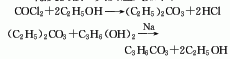
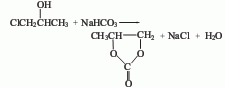
3. Chloropropanol method.
4. Propylene oxide and carbon dioxide Synthetic method: Carbon dioxide and propylene oxide react at 150-160°C and 5MPa to produce propylene carbonate. The finished product is obtained by fractionation under reduced pressure. The above methods have all been industrialized, but the first three methods have high production costs and poor product quality, so they are gradually replaced by the fourth method.
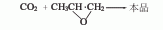
5. Propylene oxidation and carbon dioxide synthesis Law. This method is a synthetic method developed by laboratories in recent years.
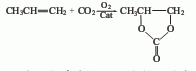
Refining method: the main impurity is water , carbon dioxide, 1,2-propanediol, allyl alcohol and propylene oxide, etc. It is generally refined by vacuum distillation. However, when used as a solvent for high-energy batteries, impurities have a significant impact. It can be dried with molecular sieves overnight, then passed through a dry molecular sieve column, fractionated twice under reduced pressure, and 2/3 of the middle distillate collected. This method can reduce the impurity content below the standard requirements.
6. Tobacco: FC, 18.
Purpose
This product is a polar solvent used as a plasticizer, spinning solvent, water-soluble dye and dispersant for plastics. It can also be used as an extraction agent for oily solvents and olefins and aromatics. Propylene carbonate is used as the battery electrolyte and can withstand harsh light, heat and chemical changes. It also has certain uses in geological mineral processing and analytical chemistry. In addition, propylene carbonate can also be used as a wood adhesive instead of phenolic resin, and is also used to synthesize dimethyl carbonate.
It is used as a solvent for synthetic fibers and other polymers, as well as an extractant and plasticizer. Propylene glycol carbonate is also used as a high dielectric constant electrochemical solution. In particular, the metal salt solution of propylene glycol carbonate is used as an aprotic electrolyte in the research of output high-voltage batteries. In addition, it is also used to separate carbon dioxide from gas mixtures and for organic synthesis. Since propylene glycol carbonate can stably dissolve free radicals, it is effectively used as a solvent for ESR (electron spin resonance).

 微信扫一扫打赏
微信扫一扫打赏

