[English name]1,4-Benzoquinone
[Molecular formula] C6H4O2
[Molecular weight] 108.10
[CA registration number][106-51-4]
[Abbreviation and alias] p-benzoquinone
[Physical properties] Yellow powder, mp 115.7 oC, d 1.318g/cm3. It is slightly soluble in water, soluble in alcohol, ether, hot petroleum ether and alkali aqueous solutions.
[Preparation and merchandise] This reagent is widely available and sold by all major reagent companies.
【Precautions】This reagent has a pungent odor and can cause conjunctivitis, corneal ulcers and dermatitis, and in severe cases can lead to skin tissue necrosis. Use with caution.
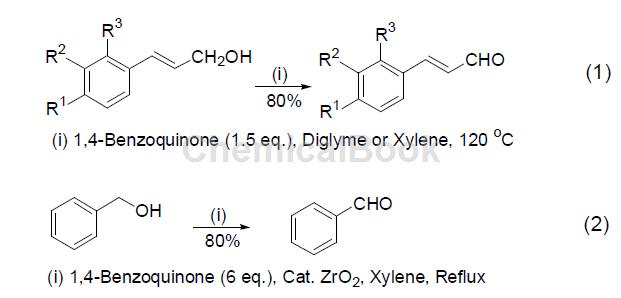
1,4-Benzoquinone is a commonly used oxidizing reagent or dehydrogenating reagent because it can be easily reduced to hydroquinone by other compounds, thus showing oxidative activity. And its own oxidation potential determines that 1,4-benzoquinone can selectively oxidize conjugated primary allyl alcohol in the coexistence of multiple alcohol compounds, such as selectively oxidizing conjugated primary allyl alcohol in the coexistence of secondary alcohols and benzyl alcohol. Oxidation of cinnamic alcohol to cinnamaldehyde (Formula 1)[1]. In addition, the oxidation reaction of primary alcohol can also be achieved by using 1,4-benzoquinone as the dehydrogenation reagent and hydrated zirconium oxide as the catalyst (Equation 2).
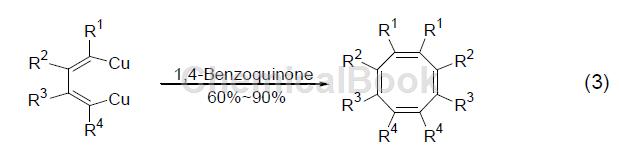
Alkenyl copper compounds are coupled with oxidants in the presence of 1,4-benzoquinone to generate conjugated diene compounds, such as dienyl copper compounds, and 1,4-benzoquinone is added for self-coupling reaction, which is successful. Cycloctatetraene derivatives (formula 3) [3] were prepared.
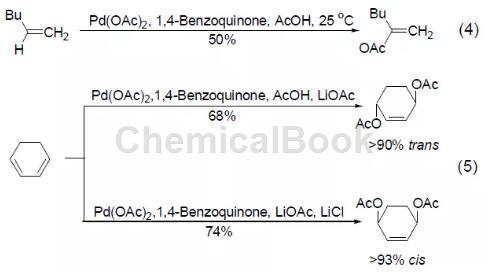
The representative use of 1,4-benzoquinone is as a co-oxidant in the palladium acetate catalytic reaction, which re-oxidizes the Pd(0) produced after reduction and elimination into Pd(II) and enters the catalytic cycle. For example, the oxidative coupling reaction between olefins and acetic acid is achieved with 0.1 times the amount of palladium acetate and 1 times the amount of 1,4-benzoquinone (Formula 4)[4]. The 1,4-benzoquinone and palladium acetate oxidation system can also achieve the conversion of 1,3-cyclohexene into 1,4-acetoxy-2-cyclohexene with a higher yield (Formula 5) [5], reaction The product will be affected by other adducts. For example, in the presence of lithium acetate, the main product of the reaction is trans-diacetoxy compound; in the presence of lithium acetate and lithium chloride, the main product of the reaction is Cis diacetoxy compound.
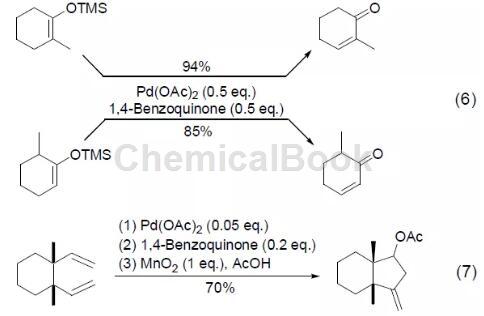
In addition, the 1,4-benzoquinone and palladium acetate oxidation system can also realize the conversion of methylsilane enol ether to conjugated enone, and the reaction has very good regioselectivity and stereoselectivity (Formula 6) [ 6]. Combining 1 part of manganese oxide reagent, 0.05 part of palladium acetate and 0.2 part of 1,4-benzoquinone can also achieve 1,5-hexadiene
Through the oxidative ring-closure reaction, cyclopentane derivatives (Formula 7) are obtained in high yields.
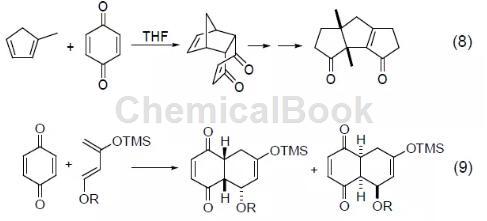
Another important type of reaction of 1,4-benzoquinone is as a dienophile reagent. Because of the electron-withdrawing effect of the carbonyl group, 1,4-benzoquinone is a good electrophile, and the Diels-Alder reaction can be easily achieved in the presence of electronegative diene substrates. A typical example is the Diels-Alder reaction of 1,4-benzoquinone and 1-methyl-1,3-cyclopentadiene in the total synthesis of Capnellene reagent, which provides a good reaction precursor for the final product ( Formula 8)[8]. In addition, there are many reports on the asymmetric Diels-Alder reaction involving 1,4-benzoquinone (Equation 9).
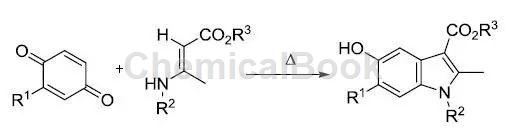
It is worth emphasizing that another important use of 1,4-benzoquinone is in the synthesis of 5-hydroxyindole derivatives (Formula 10) [10]. The reaction is very simple and has a wide substrate selectivity.

 微信扫一扫打赏
微信扫一扫打赏

