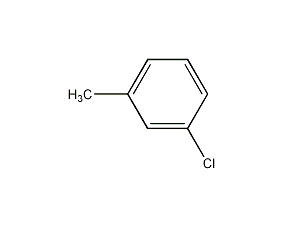
Structural formula
| Business number | 02X3 |
|---|---|
| Molecular formula | C7H7Cl |
| Molecular weight | 126.58 |
| label |
m-chlorotoluene, 1-Chloro-3-methylbenzene, 3-Chloro-1-methylbenzene, m-Chlorotoluene, 3-Chloro-1-Methylbenzene, 1-Chloro-3-Methylbenzene, Halogenated hydrocarbon solvents, aromatic compounds |
Numbering system
CAS number:108-41-8
MDL number:MFCD00000595
EINECS number:203-580-5
RTECS number:None
BRN number:1903632
PubChem number:24862373
Physical property data
1. Properties: colorless liquid with an almond-like odor.
2. Boiling point (ºC, 101.3kPa): 161.7
3. Boiling point (ºC, 13kPa): 96.3
4. Boiling point (ºC, 1.33kPa ): 43.3
5. Boiling point (ºC, 0.13kPa): 4.8
6. Melting point (ºC): -47.8
7. Relative density (g /mL, 20/4ºC): 1.0719
8. Relative density (g/mL, 30/4ºC): 1.0625
9. Relative density (g/mL, 40/4ºC ): 1.0530
10. Relative density (g/mL, 50/4ºC): 1.0438
11. Refractive index (n20ºC): 1.5224
12. Viscosity (mPa·s, 0ºC): 1.178
13. Viscosity (mPa·s, 10ºC): 1.0177
14. Viscosity (mPa·s, 20ºC): 0.877
15. Viscosity (mPa·s, 60ºC): 0.552
16. Viscosity (mPa·s, 100ºC): 0.392
17. Flash point (ºC) :50
18. Ignition point (ºC):>500
19. Heat of combustion (KJ/mol, 18.8ºC): -3749
20. Dissolution Properties: Miscible with ethanol, ether, benzene, chloroform and other organic solvents, but insoluble in water.
21. Relative density (25℃, 4℃): 1.0433
22. Refractive index at room temperature (n25): 1.406875
Toxicological data
Rat oral LD50: 3600mg/kg.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 35.97
2. Molar volume (cm3/mol): 117.6
3. Isotonic specific volume (90.2K ): 280.7
4. Surface tension (dyne/cm): 32.4
5. Dielectric constant: 2.39
6. Dipole moment (10-24cm3):
7. Polarizability: 14.26
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 70.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants.
2.This product is toxic, rat LD503600mg/kg. The operating area should be well ventilated, the equipment should be sealed, and the operators should wear protective equipment.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials. 2. This product can burn when exposed to open flames. It should be stored in a cool, ventilated warehouse, away from fire and heat sources, and stored separately from oxidants. When handling, pack and unload with care to keep the packaging intact.
Synthesis method
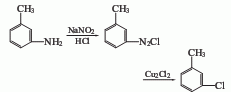
Obtained from diazotization and replacement of m-aminotoluene. Add m-aminotoluene to water, slowly add concentrated hydrochloric acid, and dropwise add sodium nitrite solution at 0-5°C. Then add diazo liquid to cuprous chloride to carry out displacement reaction. The resulting crude product is distilled to obtain the finished product. If the diazonium salt is made into zinc chloride double salt of m-toluidine diazonium salt, it is refluxed and decomposed in diethyl ether, and the reactant is cooled, washed with water and dried. After recovering the ether, perform atmospheric distillation and collect the 160-162°C fraction to obtain m-chlorotoluene. The advantage of this method is that the diazonium salt is not easily hydrolyzed to produce phenols.
Purpose
Intermediates and solvents for the manufacture of pesticides, pharmaceuticals, dyes and peroxides. Used in organic synthesis as solvent.

 微信扫一扫打赏
微信扫一扫打赏

