Background and overview[1-2]
Norfenefrine hydrochloride, also known as norfenefrine hydrochloride, scientific name m-hydroxyphenylethanolamine hydrochloride, English name NorfenefrineHydrochloride, is an adrenomimetic drug that can excite nerves similar to epinephrine. Drug. Adrenergic receptors are divided into two types: alpha-receptors and beta-receptors. α-Receptors mainly exist on effector cells of blood vessels such as glands, skin, mucous membranes and internal organs. When α-receptors are excited, the main manifestations are the contraction of blood vessels in the skin, mucous membranes and visceral blood vessels, which increases peripheral resistance and increases blood pressure. Drugs that excite α-receptors are used clinically to increase blood pressure and fight shock. Norphen hydrochloride is clinically used to excite α-receptors, constrict blood vessels, increase peripheral resistance, and increase blood pressure. There have been many reports on the synthesis of this compound. The earliest synthesis of norphenylephrine hydrochloride can be seen in the US patent US2312916 in 1943. The patent uses m-hydroxyacetophenone as raw material and is obtained through bromination, hexamethylenetetramine amination, and palladium hydrocarbon reduction. The yield of this synthesis method is Low, and the resulting product is not easy to purify. In the early days, there was also a patent for using m-hydroxyacetophenone as raw material to obtain norphenylephrine hydrochloride through a five- or six-step reaction.
Preparation[1]
The preparation method of norphenylephrine hydrochloride is carried out according to the following steps:
a. Use sodium methoxide or potassium hydroxide as the base, slowly add nitromethane dropwise at 0°C, stir for 15-60 minutes at 0°C to form a light yellow negative ion suspension, and then add m-hydroxybenzaldehyde first After being dissolved in nitromethane, slowly drop into the suspension, raise to room temperature, stir, add saturated ammonium chloride solution to terminate the reaction, extract the reaction solution with ethyl acetate, combine the organic phases, wash with saturated brine, and filter with anhydrous After drying over sodium sulfate and concentrating, a light yellow oil was obtained, which was purified by column chromatography to obtain a light yellow solid intermediate product 3-(1-hydroxy-2-nitro)ethylphenol. The reaction formula is:
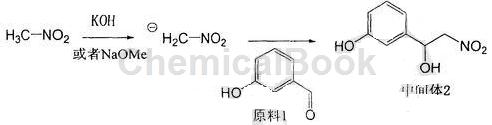
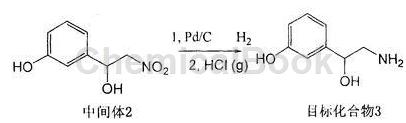
b. Dissolve the intermediate product 3-(1-hydroxy-2-nitro)ethylphenol in anhydrous methanol, add a palladium carbon catalyst, catalytically hydrogenate at room temperature and normal pressure, and stir for 10-20 hours. It was filtered through diatomaceous earth and the filtrate was concentrated to obtain the crude product.
Main reference materials
[1] CN200610070907.0 Preparation method of norphenylephrine hydrochloride
[2] Separation of norphenylephrine hydrochloride enantiomers by carboxymethyl-β-cyclodextrin chiral mobile phase additive method

 微信扫一扫打赏
微信扫一扫打赏

