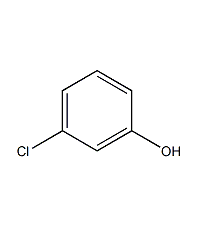
Structural formula
| Business number | 02X5 |
|---|---|
| Molecular formula | C6H5ClO |
| Molecular weight | 128 |
| label |
3-Chloro-1-hydroxybenzene, 3-Hydroxychlorobenzene, m-chloro(phenol)phenol, m-Hydroxychlorobenzene, m-Chlorophenol, m-chlorophenol, 3-chlorophenol, 3-Chloro-1-Hydroxybenzene, 3-Chloro-Phenol, 3-Hydroxychlorobenzene, m-Chlorophenic acid |
Numbering system
CAS number:108-43-0
MDL number:MFCD00002256
EINECS number:203-582-6
RTECS number:SK2450000
BRN number:1634401
PubChem number:24862755
Physical property data
1. Characteristics: white to yellow crystals with a phenol smell. [1]
2. Melting point (℃): 32~33[2]
3. Boiling point (℃) :214[3]
4. Relative density (water=1): 1.268 (25℃)[4]
5. Saturated vapor pressure (kPa): 0.13 (44.2℃)[5]
6. Critical pressure (MPa): 4.75~5.31[6]
7. Octanol/water partition coefficient: 2.47~2.5[7]
8. Flash point (℃): 91[8]
9. Explosion upper limit (%): 8.8[9]
10. Explosion lower limit (%): 1.7 [10]
11. Solubility: slightly soluble in water, soluble in ethanol, ether, alkali, and easily soluble in benzene. [11]
12. Refractive index at room temperature (n25): 1.556540
13. Relative density (25℃, 4℃): 1.213778
14. Relative density (20℃, 4℃): 1.21825
15. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -153.7
16. The liquid phase standard claims heat (enthalpy) ( kJ·mol-1): -189.3
17. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -206.4
Toxicological data
1. Acute toxicity: rat oral LD50: 570mg/kg; rat intraperitoneal LD50: 355mg/kg; rat subcutaneous LD50: 1390mg/kg; mouse oral LD50: 521mg/kg;
p>
2. Chronic toxicity/carcinogenicity: Mouse skin contact TDLo: 6000mg/kg/15W-I;
3. Mutagenicity: Microbial Salmonella typhimurium mutation: 10μg/plate ;
4. It is irritating to human skin and eyes, and its dust is irritating to human respiratory system. It is a less toxic substance.
5. Acute toxicity[12] LD50: 570mg/kg (rat oral)
6. Irritation No information available
7. Mutagenicity[13] Microbial mutagenicity: Salmonella typhimurium 10μg/dish.
8. Carcinogenicity[14] Mice.Minimum toxic dose to skin (TDLo): 6000mg/kg/15 weeks (intermittent), suspected tumor-causing agent, causing skin tumors.
Ecological data
1. This substance is harmful to the environment and can cause pollution to water bodies and soil, especially to molluscs, fish and mammals.
2. Ecotoxicity[15] LC50: 10mg/L (48h) (rainbow trout); 3.8mg /L (48h) (Medaka)
3. Biodegradability No data available
4. Non-biodegradability [16] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 , the degradation half-life is 12h (theoretical).
5. Other harmful effects[17] This substance is harmful to the environment. It can cause pollution to water bodies and soil, and can cause serious harm to molluscs, fish and mammals in particular.
Molecular structure data
1. Molar refractive index: 33.02
2. Molar volume (cm3/mol): 99.8
3. Isotonic specific volume (90.2K ): 258.1
4. Surface tension (dyne/cm): 44.7
5. Polarizability: 13.09
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 74.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[18] Stable
2. Incompatible substances [19] Strong oxidants, strong acids, acid chlorides, acid anhydrides
3. Conditions to avoid contact[ 20] Heating
4. Polymerization hazard[21] No polymerization
5. Decomposition products[22] Hydrogen chloride
Storage method
Storage Precautions[23] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
There are three preparation methods:
Dichlorosulfuryl is used as a chlorinating agent to chlorinate phenol
After heating and melting the phenol, the temperature drops to 40°C, slowly add dichlorosulfuryl, which takes about 40 to 50 hours, while stirring and dripping Add dichlorosulfonyl. After the addition of dichlorosulfonyl is completed, raise the temperature to 30~40℃ and keep it for several hours. Cool to room temperature, wash with water, and then wash with sodium carbonate and water to obtain the ortho and para products. Then distill under reduced pressure, separate, cool and crystallize to obtain the product.
Diazotization of p-chloroaniline
Add p-chloroaniline into the reactor, add water, sodium nitrite and sulfuric acid, conduct diazotization at about 0℃ to obtain diazonium salt, and then add the diazo The salt is hydrolyzed to obtain the product.
Direct chlorination with chlorine
Heat and melt the phenol, add iron powder and other catalysts, control a certain temperature, and pass in chlorine. The amount of chlorine should be controlled. After the chlorine is passed, keep it warm for several hours and cool. Perform post-processing such as washing, and then distill under reduced pressure to separate o-chlorophenol and p-chlorophenol, and then cool to obtain the product.
In summary, among the three methods, the p-chloroaniline diazotization method is rarely used in industry due to its complicated process, high cost, and large amount of waste water; the dichlorosulfuryl method in the presence of iron catalyst The chlorination reaction takes a long time, the production capacity is low, and the cost of using dichlorosulfonyl is high. However, this method has a higher para-isomer yield, reaching 70% to 75%. Currently, many factories use this method; Recently, the direct chlorination method using chlorine gas in my country has a simple process, no solvent, low investment, and low cost. It is an economical and reasonable process route. However, the production of p-chlorophenol by this method causes serious corrosion to the equipment.
Purpose
1. This product can be used in organic synthesis. Its isomers o-chlorophenol and p-chlorophenol occupy an important position in the pesticide, dye, and pharmaceutical industries. Using it to replace o-chlorophenol or p-chlorophenol can develop products with excellent performance. Pesticides, medicines and dye products, the literature has also reported the synthesis of biologically active antibiotic drugs and agricultural fungicides using m-chlorophenol as raw material, and m-chlorophenol is also used in microscopic analysis.
2. Used as an intermediate in organic synthesis. [24]

 微信扫一扫打赏
微信扫一扫打赏

