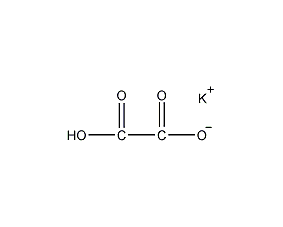
Structural formula
| Business number | 03L9 |
|---|---|
| Molecular formula | C2HKO4 |
| Molecular weight | 128.13 |
| label |
Oxalic acid monopotassium salt, potassium bioxalate, aliphatic compounds |
Numbering system
CAS number:127-95-7
MDL number:MFCD00036407
EINECS number:None
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Physical property data
1. Properties: colorless crystals
2. Density (g/mL, 25/4℃): 2.0
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): Uncertain
5. Boiling point (ºC, 0.67kpa or 5 mmHg): 148
6. Refractive index: Uncertain
7. Flash point (ºC): Uncertain
8. Specific rotation (ºC) : Uncertain
9. Autoignition point or ignition temperature (ºC) is uncertain
10. Vapor pressure (kPa, 25ºC): Uncertain
11 . Saturated vapor pressure (kPa, 60ºC): Uncertain
12. Heat of combustion (KJ/mol): Uncertain
13. Critical temperature (ºC): Uncertain
p>
14. Critical pressure (KPa): Uncertain
15. Log value of oil-water (octanol/water) partition coefficient: Uncertain
16. Explosion upper limit (%, V/V): Uncertain
17. Lower explosion limit (%, V/V): Uncertain
18. Solubility: Easily soluble in water.
Toxicological data
1. Acute toxicity: Oral TDLO for women: 100mg/kg
Oral LDLO for women: 660mg/kg
Oral TDLO for women: 400mg/kg
Intravenous injection of TDLO for men: 1071mg/kg
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 77.4
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 87.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters : 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Easily soluble in water and cannot be recrystallized from water.
Storage method
None
Synthesis method
Dissolve 12.6g oxalic acid dihydrate (0.1mol) in 300mL water. Dissolve 18.4g potassium oxalate monohydrate (0.1mol) in 100mL water. Then add the potassium oxalate aqueous solution to the oxalic acid aqueous solution at 3°C and leave it at 0°C for 24 hours. The precipitated crystals are potassium hydrogen oxalate monohydrate.
Purpose
None

 微信扫一扫打赏
微信扫一扫打赏

