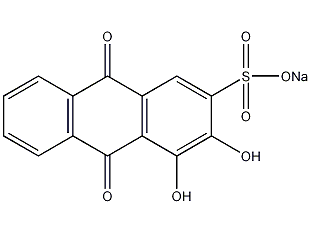
Structural formula
| Business number | 03MF |
|---|---|
| Molecular formula | C14H7NaO7S |
| Molecular weight | 342.26 |
| label |
9,10-Dihydro-3,4-dihydroxy-9,10-dioxo-2-anthracenesulfonic acid monosodium salt, alizarin sodium sulfonate, medium red 3, alizarin red, Sodium 1,2-dihydroxyanthraquinone-3-sulfonate, 1,2-Dihydroxyanthraquinone-3-sulfonate sodium salt, 3,4-Dihydroxy-9,10-dioxo-2-anthracenesulfonic acid sodium salt,, Alizarin carmine, Alizarin red, Alizarin sodium monosulfonate, Alizarin sulfonate sodium, Alizarinsulfonic acid sodium salt, Sodium alizarinsulfonate, Reagents for histochemical research |
Numbering system
CAS number:130-22-3
MDL number:MFCD00013049
EINECS number:204-981-8
RTECS number:CB1095300
BRN number:4117090
PubChem number:24890988
Physical property data
1. Properties: orange-yellow or yellow-brown powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Not determined
5. Boiling point (ºC, normal pressure): Not determined
6. Boiling point (ºC, 5.2kPa): Not determined Determined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturation Vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% , V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, slightly soluble in ethanol , insoluble in benzene and chloroform.
Toxicological data
1. Acute toxicity: Mouse intravenous LD5O: 70mg/kg
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 16
6. Topological molecular polar surface area (TPSA): 132
7. Number of heavy atoms: 23
8. Surface charge: 0
9. Complexity: 599
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. The aqueous solution turns light yellowish brown, turns yellow after adding acid, and turns blue-purple after adding alkali. It forms red-purple or red-orange complexes with aluminum, cobalt, beryllium, zirconium, thorium and other metal ions. A certain amount of fluoride can make zirconium, thorium and other complexes fade.
Storage method
Keep sealed.
Synthesis method
Introduction to the production method
1. Add alizarin to 15% fuming sulfuric acid, sulfonate it at 105-110℃, keep it warm for 3 hours, cool it, pour it into distilled water, add sodium chloride to salt out at 60℃, and heat to 70℃ , leave it overnight and filter. After washing with 10% sodium chloride solution, dissolve it in distilled water, place it, and filter. Soak the crystals in a solution of ethanol and hydrochloric acid (9:1), stir continuously, and leave overnight. The crystals are dried below 80°C. Purification: Dissolve the alizarin red obtained by the method in hot water, add activated carbon to decolorize, and filter. Stir the filtrate while adding ammonia water until a small amount of crystals precipitate. Stir again for 20 minutes, leave it overnight, and filter. The product will be at 70-80°C. dry.
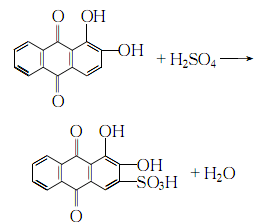
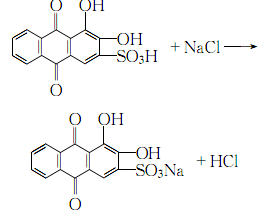
2.Heat the crude alizarin red Dissolve in water, add activated carbon for decolorization, slowly add filtered clear industrial ammonia to the filtrate while stirring until a small amount of crystals precipitate, continue stirring for 20 minutes, let it stand for crystallization, and filter Afterwards, the crystals are dried at 70-80°C to obtain refined alizarin red.
Purpose
1. Determination of fluoride. Acid-base indicator. Alizarin red can form a stable red complex with many metal ions and is often used as a metal indicator in analysis; a color developer for the photometric determination of metal ions; often used as a metal indicator in analysis; a photometric method for the determination of metal ions. Color developer; acid-base indicator, the first color change range is PH value 3.7 (yellow) ~ 5.2 (purple), the second color change range PH value is 10.1 (purple) ~ 12.0 (light yellow); used for polarographic determination of metal ions.
2.Used as an acid-base indicator, with a pH value of 3.7 (yellow) to 5.2 (purple). Used as a chromogen for the photometric determination of fluoride, aluminum, beryllium, germanium, indium and cobalt. It is also used as an indicator for the determination of rhenium, thorium, scandium and other metals by the E-Ben-T-A method.

 微信扫一扫打赏
微信扫一扫打赏

