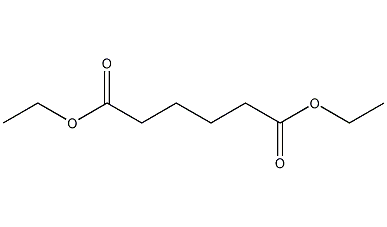
Structural formula
| Business number | 03TW |
|---|---|
| Molecular formula | C10H18O4 |
| Molecular weight | 202.25 |
| label |
Ethyl fertilizer, Ethyl adipate, diethyl fertilizer, 1,6-Diethyl hexanedioate, Diethyl1,6-hexanedioate, Hexanedioic acid diethyl ester, C2H5OOC(CH2)4COOC2H5, aliphatic compounds |
Numbering system
CAS number:141-28-6
MDL number:MFCD00009215
EINECS number:205-477-0
RTECS number:AV1100000
BRN number:1780035
PubChem number:24854754
Physical property data
1. Properties: colorless oily liquid.
2. Density (g/mL, 20/4℃): 1.0076
3. Refractive index (20ºC): 1.4272
4. Melting point (ºC ): -19.8
5. Boiling point (ºC, 101.3kPa): 245
6. Boiling point (ºC, 1.729kPa): 127
6. Flash Point (ºC): 110
7. Solubility: Soluble in ethanol and other organic solvents, insoluble in water.
8. Relative density (25℃, 4℃): 0.997430
Toxicological data
1. Acute toxicity: Mouse oral LD50: 8100 mg/kg; Mouse abdominal LD50: 2190 mg/kg.
2. Teratogenicity: Mouse abdominal cavity: 1100 mg/kg
3. Reproductive toxicity: rat abdominal TDLo: 837 mg/kg;
4. Strongly irritating, high concentrations can seriously damage mucous membranes, upper respiratory tract, eyes, and skin. Contact may cause coughing, headache, nausea, and vomiting.
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 51.92
2. Molar volume (cm3/mol): 200.5
3. Isotonic specific volume (90.2K ): 479.4
4. Surface tension (dyne/cm): 32.6
5. Polarizability (10-24cm3): 20.58
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 9
5. Topological molecular polar surface area (TPSA): 52.6
6. Number of heavy atoms: 14
7. Surface charge: 0
8. Complexity: 157
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters :0
11. Unsure.Number of substereocenters: 0
12. Determined number of stereocenters of chemical bonds: 0
13. Uncertain number of stereocenters of chemical bonds: 0
14. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure. Incompatible materials: strong oxidants, strong acids, and strong bases. It is flammable and can cause combustion when exposed to open flames or high heat.
Storage method
Keep sealed.
Synthesis method
1. Obtained from the esterification of adipic acid and ethanol in the presence of concentrated sulfuric acid.
2. Preparation method:
In a reaction bottle equipped with a distillation device, add 22g (0.15mol) of adipic acid (2) and 42g (0.9mol) of absolute ethanol ), 30mL of toluene, one grain of zeolite, 0.5mL of sulfuric acid. Add 30g anhydrous sodium carbonate to the receiving bottle. The reactants are slowly heated and distilled. The ternary azeotrope of ethanol, benzene and water evaporates at 75°C. When the gas phase temperature reaches 78°C, heating is suspended. Pour the clear liquid in the receiving flask into the distilling flask and continue the esterification reaction. Heat slowly to distill. When the gas phase temperature reaches 78°C, heating is suspended. Pour the clear liquid in the receiving flask into the distilling flask and continue the esterification reaction. Heat slowly to distill. When the gas phase temperature reaches 70~80℃, stop distillation. The residue was re-distilled, ethanol and toluene were first evaporated, and then distilled under reduced pressure. The fraction at 133°C/2.67kPa was collected to obtain 29g of diethyl adipate (1), with a yield of 97%. [1]
Purpose
It is used as a solvent and organic synthesis intermediate. Hexanediol can be prepared by hydrogenation and reduction. It can also be used in daily chemical industry and food industry. Used as a plasticizer for cellulose acetate, cellulose acetate butyrate and cellulose nitrate.

 微信扫一扫打赏
微信扫一扫打赏

