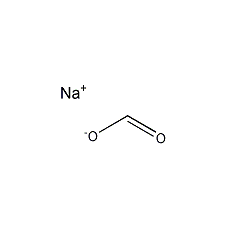
Structural formula
| Business number | 03U2 |
|---|---|
| Molecular formula | CHNaO2 |
| Molecular weight | 68.01 |
| label |
Sodium formate dihydrate, Formic acid sodium salt, sodium formate, Sodium formate dihydrate, Sodium fulminate, Sodium formate dihydrate, Sodium formate dihydrate, Formic acid sodium salt, preservative, antiseptic pesticides, food additives |
Numbering system
CAS number:141-53-7
MDL number:MFCD00013101
EINECS number:205-488-0
RTECS number:LR0350000
BRN number:3595134
PubChem number:24886222
Physical property data
1. Physical property data:
1. Properties: white granular or crystalline powder. It is hygroscopic and has a slight formic acid smell.
2. Density (g/mL, 25/4℃): 1.92
3. Melting point (℃): 253
4. Boiling point (ºC, Normal pressure): 360 ºC
5. Solubility: Soluble in water and glycerol, slightly soluble in ethanol, insoluble in ether.
Toxicological data
2. Toxicological data:
1. Acute toxicity: Mouse oral LC50: 11200 mg/kg;
Mouse intravenous LC50: 807 mg/kg;
Mouse not reported LC50: 2500 mg/kg;
Rabbit intravenous LDLo: 1250 mg/kg.
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None yet
Compute chemical data
IV. Calculated chemical data:
1. Number of hydrogen bond donors: 0
2. Number of hydrogen bond acceptors: 2
3. Number of rotatable chemical bonds: 0
4. Topological molecular polar surface area (TPSA): 40.1
5. Number of heavy atoms: 4
6. Surface charge : 0
7. Complexity: 13.5
8. Number of isotope atoms: 0
9. Determine the number of atomic stereocenters: 0
10. The number of uncertain atomic stereocenters: 0
11. The number of determined chemical bond stereocenters: 0
12. The number of uncertain chemical bond stereocenters: 0
13. Number of covalent bond units: 2
Properties and stability
1. Stable under normal temperature and pressure.
Incompatible materials: strong oxidants.
The aqueous solution is neutral, with a pH value of approximately 7
2.This product is toxic. For protection methods, see formic acid. Generally, intermediates are used for their own use.
Storage method
1. Store in a sealed and dry container. It can be packed in plastic bags lined with plastic bags or woven bags coated. Storage and transportation in accordance with general chemical regulations.
Synthesis method
1. Industrially, it is obtained by reacting carbon monoxide and sodium hydroxide at 150-170℃ and about 2MPa. In fact, the production process of sodium formate is part of the production of oxalic acid, and the concentration of sodium hydroxide solution used for the absorption reaction is 25%-30%. Sodium formate can also be prepared by reacting formic acid with sodium oxyoxide or sodium bicarbonate. Raw material consumption quota: carbon monoxide (>28%) 1630kg/t, caustic soda (>30%) 2160kg/t.
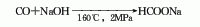
2. Pentaerythritol by-product.
3. It is formed by the reaction of carbon monoxide and sodium hydroxide at 160℃ and 2MPa.
4.Produced by the reaction of carbon monoxide and sodium hydroxide. Or it can be obtained by reacting formic acid with sodium hydroxide or sodium bicarbonate.
5. React with sodium bicarbonate solution and formic acid, remove impurities, concentrate and crystallize, and then wash with ethanol to obtain:
span>
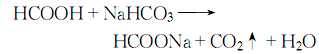
Purpose
1. Mainly used to produce formic acid, oxalic acid and insurance powder.
2. Used as reagent, disinfectant and mordant for determination of phosphorus and arsenic.
3. Preservatives. Has diuretic effect. EEC countries are allowed to use it, but the UK is not allowed to use it.
4. It is an intermediate in the production of formic acid and oxalic acid, and is also used in the production of dimethylformamide, etc. Also used in medicine, printing and dyeing industries. It is also a precipitant for heavy metals.
5. Used in alkyd resin coatings, plasticizers, high explosives, acid-resistant materials, aviation lubricants, and adhesive additives.
6.Heavy metal precipitant can form trivalent metal complex ions in solution. Reagents for the determination of phosphorus and arsenic. Also used as a disinfectant, astringent, and mordant. It is also an intermediate in the production of formic acid and oxalic acid, and is used in the production of dimethylformamide, etc.
7.Used for nickel-cobalt alloy electrolyte plating.

 微信扫一扫打赏
微信扫一扫打赏

