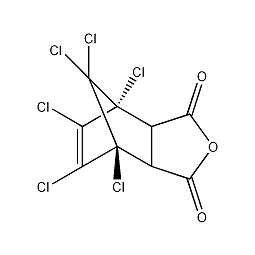
Structural formula
| Business number | 037V |
|---|---|
| Molecular formula | C9H2Cl6O3 |
| Molecular weight | 370.83 |
| label |
1,4,5,6,7,7-hexachlorobicyclo(2,2,1)-5-heptene-2,3-dicarboxylic anhydride, Hexachloronorbornedioic anhydride, Hexachloromethylenetetrahydrophthalic anhydride, 1,4,5,6,7,7-hexachloro-bicyclo(2,2,1)-5-Gene-2,3- dicarboxylic acid anhydride, Hexachloro endo methylene-tetrahydrophthalic anhydride, flame retardant curing agent, Semiconductor encapsulants |
Numbering system
CAS number:115-27-5
MDL number:MFCD00080438
EINECS number:204-077-3
RTECS number:RB9080000
BRN number:92693
PubChem number:24846601
Physical property data
1. Properties: White crystalline solid
2. Density (g/mL, 20℃): 1.73
3. Relative vapor density (g/mL, air=1 ) Undetermined:
4. Melting point (ºC): 240~241
5. Boiling point (ºC , normal pressure): not determined
6. Boiling point (ºC, KPa): not determined
7. Refractive index (n20/D): Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (ºC): Undetermined
10. Autoignition point or ignition Combustion temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 38ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: slightly soluble in water and hydrolyzed into acid, solubility (g/100g solvent): acetone 127.0, benzene 40.4 , carbon tetrachloride 6.7, hexane 4.5
Toxicological data
1. Acute toxicity: Oral LD5O in rats: 2300mg/kg
Inhalation LC5O in rats: >1gm/m3
Oral LD5O in mice: 2400mg/kg
Cats inhaled LC5O: >1gm/m3
RabbitsInhaled LC5O: >1gm/m3
2. Other multiple dose toxicity: Rat inhalation TCLO: 51500mg/m3/30D-I
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 67.99
2. Molar volume (cm3/mol): 186.2
3. Isotonic specific volume (90.2K): 531.1
4. Surface tension (dyne/cm): 66.1
5. Polarizability (10-24cm3): 26.95
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 43.4
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 498
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 4
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
White crystal. Solubility (g/100g): benzene 40.4, hexane 4.5, acetone 127.0, carbon tetrachloride 6.7, slightly soluble in water and hydrolyzed into chlorobridge acid. This product contains a large amount of chlorine compounds. Care should be taken to avoid inhaling the dust and not allowing the dust to stick to the skin.
Storage method
Stored in a cool, ventilated warehouse, protected from heat, moisture and light.
Synthesis method
Obtained from the reaction of hexachlorocyclopentadiene and maleic anhydride. Mix hexachlorocyclopentadiene and maleic anhydride in the solvent chlorobenzene at a molar ratio of 1:1.1. After reacting at 140-145°C for 7-8 hours, add the reaction product to water for hydrolysis. The chlorine obtained by hydrolysis at 70°C The bridge acid becomes an oily liquid, which is miscible with water when heated to 96-97°C. After cooling, it becomes a chlorobridge acid containing a crystal water (Het acid [115-28-6]). If the reaction product is crystallized with hot water and dilute acetic acid, the monohydrate is dried at 100-105°C to obtain chloro-bridged anhydride.
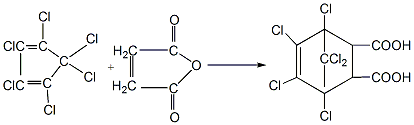
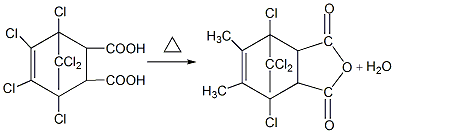
Purpose
Chloro-bridged anhydrides and chloro-bridging acids are both reactive flame retardants, suitable for unsaturated polyester, polyurethane and epoxy resins, and are also used as epoxy resin curing agents. The heat distortion temperature of epoxy resin products cured by this product is about 180°C, and it has good drug resistance. Chlorobridge acid is also used as a dye intermediate and pesticide. Also used as a flame retardant for fabrics. Used as a flame retardant curing agent for epoxy resins. While making the resin flame retardant, the resin is cured. Flame-retardant epoxy resin is used as a sealant for semiconductors, etc. Used as a curing agent for epoxy resin, the reference dosage is 100 to 110 parts by mass. Curing conditions: 100℃/2h+160℃/4h or 120℃/2h+180℃/4h or 100℃/1h+160℃/4h+200℃/1h. The cured product has excellent flame retardancy, electrical properties and mechanical properties, and excellent chemical resistance. The heat distortion temperature is 181℃. Chloro-bridged anhydrides and chloro-bridging acids can also be used as reactive flame retardants for polyester, polyurethane and unsaturated polyester. Chloride is a dye intermediate and pesticide and a flame retardant for fabrics.

 微信扫一扫打赏
微信扫一扫打赏

