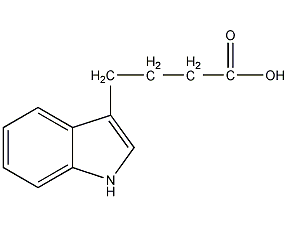
Structural formula
| Business number | 03P1 |
|---|---|
| Molecular formula | C12H13NO2 |
| Molecular weight | 203 |
| label |
3-Indolylbutyric acid, 4-(indol-3-yl)butyric acid (IUPAC), Indole-3-butyric acid, Indolebutyric acid, 3-indolebutyric acid, 4-(3-indolyl)butyric acid, 3-Indolebutyric acid (IBA), Azindylbutyric acid, Indolebutyric acid, Plant Growth Regulator |
Numbering system
CAS number:133-32-4
MDL number:MFCD00005664
EINECS number:205-101-5
RTECS number:NL5250000
BRN number:171120
PubChem number:24896067
Physical property data
1. Physical property data
1. Properties: The pure product is a white crystalline solid.
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 124~125
5. Boiling point (ºC, 0.67kpa or 5 mmHg) : Uncertain
6. Refractive index: Uncertain
7. Flash point (ºC): Uncertain
8. Specific rotation (ºC): Uncertain
9. Autoignition point or ignition temperature (ºC) is uncertain
10. Vapor pressure (kPa, 25ºC): <10×10-6
sup>
11. Saturated vapor pressure (kPa, 60ºC): Uncertain
12. Heat of combustion (KJ/mol): Uncertain
13. Critical temperature (ºC): 50.3
14. Critical pressure (KPa): Uncertain
15. Log value of oil-water (octanol/water) partition coefficient: Uncertain
p>
16. Explosion upper limit (%, V/V): Uncertain
17. Explosion lower limit (%, V/V): Uncertain
18. Dissolution Properties: Hardly soluble in water, solubility in water is 0.25g/L at 20°C. Easily soluble in benzene and soluble in other organic solvents.
Toxicological data
Acute toxicity data:
Rat oral LD: >500mg/kg
Mouse oral LD50: 100mg/kg
Mouse intraperitoneal cavity LD50: 100mg/kg
Mutation data:
Mold-Aspergillus nidulans gene conversion and mitosis: 1mmol/L
Mold-Aspergillus nidulans sex chromosomes Loss: 1mmol/L
Human leukocytes: 100nmol/L
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 58.90
2. Molar volume (cm3/mol): 162.3
3. Isotonic specific volume (90.2K ): 448.6
4. Surface tension (3.0 dyne/cm): 58.3
5. Polarizability (0.5 10-24cm 3): 23.35
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.3
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 53.1
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 230
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
1. Obtained from the condensation of indole and γ-butyrolactone at the reflux temperature of tetralin. Or by reacting indole with Grignard reagent and α-chloropropionitrile, 3-butyronitrile indole is obtained, which is then hydrolyzed by NaOH and reacted with hydrochloric acid.
2.Add indole, γ-butyrolactone and potassium hydroxide to tetralin, stir and heat to dissolve , reflux and dehydrate, add xylene and hot water to dissolve after slightly cooling, and separate the liquids while hot. After the water layer is cooled, it is neutralized with hydrochloric acid to obtain a crude product, which is then recrystallized with benzene to obtain indolebutyric acid.
Purpose
1. It is a broad-spectrum indole plant growth regulator and a good rooting agent, which can promote the rooting of herbaceous and woody ornamental plant cuttings. .
2.Plant growth regulator. It is often used for root soaking and transplanting of woody and herbaceous plants, which can accelerate root growth and increase the percentage of plant rooting. It can also be used for seed soaking and seed dressing of plant seeds, which can increase the germination rate and survival rate.

 微信扫一扫打赏
微信扫一扫打赏

