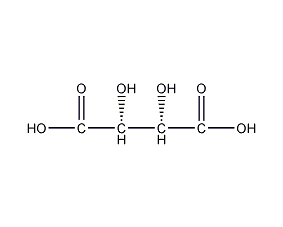
Structural formula
| Business number | 03P2 |
|---|---|
| Molecular formula | C4H6O6 |
| Molecular weight | 150.09 |
| label |
Gratic acid; racemic tartaric acid; dihydroxysuccinic acid; racemic tartaric acid, 2,3-dihydroxysuccinic acid, Racemic acid, Racemic tartaric acid, Parratartaric acid, 2,3-Dihydroxysuccinic acid, Tartaric acid, food and feed additives, food sour agent, food additives, acidity regulator, acidic solvent |
Numbering system
CAS number:133-37-9
MDL number:MFCD00071626
EINECS number:205-105-7
RTECS number:None
BRN number:1725148
PubChem number:24845619
Physical property data
1. Properties: white crystal, odorless, sour taste. It is an equal mixture of left-handed and right-handed tartaric acid. 2. Solubility: Easily soluble in water and ethanol, slightly soluble in ether and chloroform. Insoluble in benzene. 3. Relative density (d254) : 1.788 (monohydrate). 4. Melting point (℃): 206 (decomposes at 210℃), 203.4 (monohydrate). 5. Flash point: 2106. Boiling point (ºC): 399.3
Toxicological data
Acute toxicity: LC50: 4360mg/Kg (orally administered to mice) Irritating
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 26.69
2. Molar volume (cm3/mol): 79.5
3. Isotonic specific volume (90.2K): 263.1
4. Surface tension (3.0 dyne/cm): 119.4
5. Polarizability (0.5 10 -24cm3): 10.58
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -1.9
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 6
p>
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 115
p>
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 134
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1.This product has low toxicity. It is highly acidic and corrosive to teeth. It is reported that when operators work in an environment with a concentration of 1.1mg/m3, they will suffer tooth damage and cause gastritis. Therefore, the operation site must have good ventilation conditions and the production equipment must be leak-free.
2. Exist in tobacco leaves.
Storage method
1. Storage should be kept sealed.
2. Packed in woven bags or sacks lined with plastic bags, with a net weight of 25kg or 50kg per bag. Keep away from oxidants and fire sources, and cannot be stored together with liquid alkali. Pay attention to rain and moisture, and store in a dry and cool place. Store and transport according to general chemical regulations.
Synthesis method
There are four industrial production methods of tartaric acid: extraction, chemical synthesis, semi-synthesis and fermentation.
1. Extraction method
Add 5 parts of crude tartar and 1 to 1.5 parts of concentrated hydrochloric acid to 100 parts of water, heat to dissolve, and filter while hot. After the filtrate is cooled, potassium hydrogen tartrate crystals precipitate. Combine 20 parts of potassium hydrogen tartrate and 100 parts of water at 100°C, slowly add calcium carbonate until the pH value is 7.0, then add calcium chloride to obtain calcium tartrate, filter and wash with cold water. Add 4 times the amount of water to the calcium tartrate precipitate, then slowly add sulfuric acid to acidolyze, and filter to remove the precipitate. The filtrate was decolorized with activated carbon at 60~70°C, filtered and concentrated under reduced pressure at 80°C. Cool, crystallize, and finally recrystallize with water in a non-ferrous container to obtain the finished product.
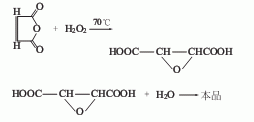
2. Chemical synthesis method
Using maleic acid and hydrogen peroxide as raw materials, it is converted into epoxy succinic acid at 70℃, and then hydrolyzed at 100℃ That is, DL-tartaric acid is obtained.
3. Semi-synthetic method
The semi-synthetic method is a combination of chemical synthesis method and enzymatic method (fermentation method), that is, epoxysuccinic acid is first prepared by the above-mentioned synthesis method, and then The ring-opening enzyme contained in Nocardia succinicus converts it into dextrorotatory L(+) tartaric acid.
4. Maleic anhydride oxidation method: Maleic anhydride reacts with hydrogen peroxide solution under the action of tungstic acid to generate epoxy succinic acid, which is then hydrolyzed to obtain tartaric acid, which is then cooled, crystallized, separated and dried to obtain pure product.
5. The tartar method uses crude tartar when making wine as raw material, processes it with lime milk to form calcium tartrate, and then acidifies it with sulfuric acid.
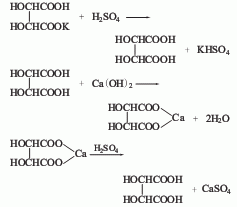
6.D type mainly uses acid hydrolysis method. The particles that crystallize outside the wine fermentation tank are potassium hydrogen tartrate. After adding hydrochloric acid to dissolve it, separate the potassium chloride, and then react with calcium carbonate to precipitate calcium tartrate. After washing with water, add sulfuric acid to remove the precipitated calcium sulfate, decolorize with active species, evaporate under reduced pressure, concentrate and crystallize.
7. It is obtained by oxidation with fumaric acid or maleic acid. It can also be obtained by reacting glyoxal with hydrocyanic acid and then acid hydrolysis.
Purpose
1. As a sour agent, the acidity is 1.2~1.3 times stronger than citric acid. It has high solubility and strong chelating ability for metal ions. It can be used in various foods and should be used in appropriate amounts according to production needs. It has a flavor-enhancing effect on drinkers, but is weaker than citric acid. Generally used in combination with other organic acids such as citric acid or malic acid.
2. Biochemical research. Test calcium and potassium. Determination of calcium and strontium. Masking agents for qualitative and quantitative analyses. Organic Synthesis.
3.Used as antioxidant synergist, tanning preparation, chelating agent and pharmaceutical. Widely used in medicine, food, tanning, textile and other industries. Its solubility in water is low at low temperatures and it is easy to form insoluble calcium salts, so please be careful when using it.
4.Tartaric acid is used to adjust the ph value in tartaric acid electrolyte, to dissolve manganese sulfate and for cyanide copper plating Brightener, also used as complexing agent for electroless plating.

 微信扫一扫打赏
微信扫一扫打赏

