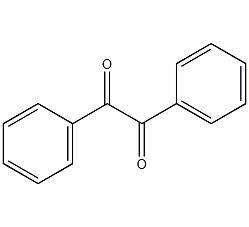
Structural formula
| Business number | 03PN |
|---|---|
| Molecular formula | C14H10O2 |
| Molecular weight | 210 |
| label |
Benzil, Dibenzoyl, Diphenylethanedione, Diphenyl-α,β-diketone |
Numbering system
CAS number:134-81-6
MDL number:MFCD00003080
EINECS number:205-157-0
RTECS number:DD1925000
BRN number:608047
PubChem number:24891811
Physical property data
1. Properties: yellow prismatic crystal powder.
2. Relative density (25℃, 4℃): 1.084
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 94~96
5. Boiling point (ºC, normal pressure): 347, 188ºC (1.6kpa)
6. Gas phase standard heat of combustion (enthalpy )(kJ·mol-1): -6882.8
7. Gas phase standard claims heat (enthalpy) (kJ·mol-1): – 55.5
8. Crystalline phase standard combustion heat (enthalpy) (kJ·mol-1): -6784.4
9. Crystalline phase standard claimed heat (Enthalpy) (kJ·mol-1): -153.9
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/water) distribution Log value of coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether, chloroform, ethyl acetate, benzene, toluene and nitrobenzene, insoluble in water.
20. Flash point (ºC): 180
Toxicological data
Skin/eye irritation data
Rabbit eye contact: 100mg/24H moderate reaction
Acute toxicity data:
Mouse oral LD50:> 3mg/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 61.24
2. Molar volume��cm3/mol): 180.3
3. Isotonic specific volume (90.2K): 473.1
4. Surface tension (3.0 dyne/cm): 47.3
5. Polarizability (0.5 10-24cm3): 24.28
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 230
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Yellow prismatic crystal. Soluble in ethanol, ether, chloroform, ethyl acetate, benzene, toluene and nitrobenzene, insoluble in water. Relative density 1.23. Melting point 95℃. Boiling point is 346~348℃. Low toxicity, LD50 (rat, oral) 2710mg/kg. Irritating.
2. Exist in smoke.
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
1.Benzoin (benzoin) is obtained from the condensation of benzoyl and sodium cyanide and then nitrated. Or oxidation of diphenylethylene to benzyl. NH4CrO3Cl and diphenylethylene were mixed at a molecular ratio of 3:1 and reacted in glacial acetic acid for 20 hours at a reaction temperature of 80°C. The yield of benzil was 80%.
2. Use dimethylamine hydrochloride chromium trioxide/silica gel carrier oxidant to oxidize benzoin system to synthesize benzoyl compounds.
① Preparation of dimethylamine hydrochloride chromium trioxide/silica gel Weigh 10g (0.1mol) chromium trioxide, dissolve it in a beaker with 10mL of water, and then add 8.2g (2.2mol) dimethylamine hydrochloride. Heat and stir in a 40°C water bath for 15 minutes, then cool to produce a large amount of orange-red solid. Reheat to dissolve, add 25mL of water, then add 50g of silica gel, stir thoroughly with a glass rod and dry under vacuum. 70g of earthy yellow carrier oxidant was obtained. 1g of this reagent is equivalent to 0.143gCrO3.
② Place 1g benzoin and 15mL cyclohexane in a three-necked round-bottomed flask equipped with electric stirring and reflux condenser, and add 8g of the above oxidant while stirring. Heat and stir in a 60°C water bath for 14 hours. Filter, wash with appropriate amount of diethyl ether, combine the filtrate, and evaporate the solvent. The crude product was recrystallized from ethanol to obtain 0.93g benzil, with a yield of 94%. 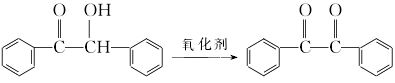
3. Preparation method:
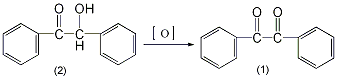
In a 250 mL reaction bottle, add 20 g (0.094 mol) of benzoin (2) and 100 mL of concentrated nitric acid, and heat the reaction in a boiling water bath for about 1.5 hours with stirring until nitrogen dioxide basically no longer escapes. Pour the reactant into 400 mL of cold water and stir until the oil solidifies into a yellow solid. Suction filter and wash with water. Recrystallize from ethanol (1g requires approximately 2.5mL of ethanol) to obtain 19g of pure bibenzoyl (1), yield 96.8%, mp94~96°C. [1]
4. Preparation method: 250mL reaction equipped with stirrer and reflux condenser In the bottle, add 0.2g copper acetate, 10g (0.125mol) ammonium acetate, 21.0g (0.1mol) benzoin (2) and 70mL 80% acetic acid aqueous solution. Heat while stirring while releasing nitrogen. Reflux the reaction for 90 minutes and cool. Sprinkle crystal seeds, leave for 1 hour, suction filter, wash with water, and dry to obtain 19 g of bibenzoyl (1), yield 90%, mp94~95°C. After the mother liquor is concentrated, 1g of product can be obtained. [2]
5.Preparation method:
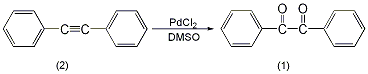
In the installation Add 178 mg (1 mmol) of diphenyl acetylene (2), 10 mL of dry DMSO, and 18 mg of palladium chloride (0.1 mmol) to a reaction flask in a magnetic stirrer and reflux condenser (installed with a calcium chloride drying tube). Slowly heat to 140°C and stir for 4 hours. After cooling, add 30 mL of water to dilute, and extract with ether three times, 30 mL each time. The organic layers were combined, washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. The solvent was evaporated and purified through a silica gel column (benzene:hexane = 1:1) to obtain 205 mg of compound (1) with a yield of 98%. [3]
Purpose
1. Organic synthesis. Pesticides. Used as photocuring agent for photosensitive adhesives and photocurable coatings.
2. Used as pharmaceutical intermediates.

 微信扫一扫打赏
微信扫一扫打赏

