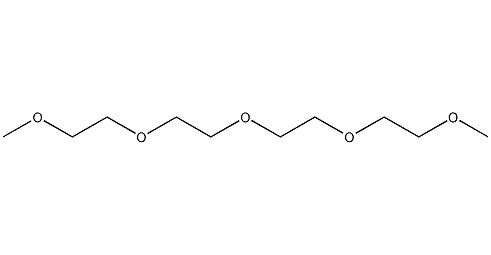
Structural formula
| Business number | 03W0 |
|---|---|
| Molecular formula | C10H22O5 |
| Molecular weight | 222.28 |
| label |
Tetraethylene glycol dimethyl ether trihydrate, Tetraethylene glycol dimethyl ether, Tetraglyme, Tetraethylene glycol dimethyl ether, 1,11-Dimethoxy-3,6,9-troxa-undecane, 1-(2-Methoxyethoxy)-2-[2-(2-methoxyethoxy)-ethoxy]-ethane, Ether and acetal solvents, Gas Chromatography Stationary Solution |
Numbering system
CAS number:143-24-8
MDL number:MFCD00008505
EINECS number:205-594-7
RTECS number:SB0400000
BRN number:1760005
PubChem number:24854465
Physical property data
1. Properties: colorless and transparent liquid.
2. Boiling point (ºC, 101.3kPa): 275.3
3. Melting point (ºC): -29.7
4. Relative density (g/mL, 20/4ºC): 1.0087
5. Relative density (25℃, 4℃): 0.991241.4
6. Refractive index (20ºC): 1.4325
7. Flash point (ºC): 140
8. Solubility: Miscible with water and various organic solvents.
9. Solubility parameter (J·cm-3)0.5: 18.067
10. van der Waals area ( cm2·mol-1): 1.804×1010
11. van der Waals volume (cm3·mol-1): 127.680
12. Liquid phase standard hot melt (J·mol-1·K-1): 400.7
Toxicological data
Acute toxicity:
Oral LD50 3850mg/kg(rat)
Irritation to skin mild unknown mg(rbt)
Irritation to eyes mild 500 mg(rbt )
Main irritant effects:
On the skin: Causes mild irritant effect.
On eyes: Causes mild irritant effect.
Sensitization: May cause sensitization through skin contact.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 57.10
2. Molar volume (cm3/mol): 225.4
3. Isotonic specific volume (90.2K ): 528.3
4. Surface tension (dyne/cm): 30.1
5. Polarizability (10-24cm3): 22.63
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: none
6. Topological molecule polar surface area 46.2
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 98
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure. Incompatible materials: strong oxidants, strong acids.
2. It is flammable and can form explosive peroxides.
Storage method
Keep sealed.
Synthesis method
1. Mainly adopts the synthesis method of diethanol monomethyl ether and thionyl chloride. Chlorine the diethanol monomethyl ether with thionyl chloride, and then convert the diethanol monomethyl ether into sodium alkoxide, then perform a condensation reaction between the two, and finally obtain it through filtration and vacuum distillation.
Purpose
1. Used as a solvent and gas chromatography stationary solution (maximum operating temperature 75°C, solvents are acetone, methanol), which can selectively retain and separate many oxygen- and nitrogen-containing functional groups as well as oxygen and nitrogen heterocyclic compounds. It also has high selectivity for aromatic compounds. It can separate normal and branched alkanes and cycloalkanes for aliphatic hydrocarbons. It is suitable for aqueous solution analysis and can directly measure water. There is less tailing of water peaks.
2.Cosmetic solvents. It is mainly used as the main solvent for cosmetics such as nail polish. It is an excellent solvent for cellulose and is called a cellosolve.

 微信扫一扫打赏
微信扫一扫打赏

