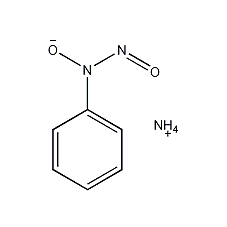
Structural formula
| Business number | 03Q3 |
|---|---|
| Molecular formula | C6H9N3O2 |
| Molecular weight | 155.16 |
| label |
N-Nitroso-N-phenylhydroxylamine ammonium salt, N-nitrosophenylhydroxylammonium, copper iron reagent, N-Hydroxy-N-nitrosanilinium salt, N-nitrosophenylhydroxylamine, N-nitrosophenylhydroxylammonium salt, copper iron spirit, Nitrosophenylammonium ammonium salt, N-nitroso-N-phenylhydroxylamine ammonium salt, aromatic compounds |
Numbering system
CAS number:135-20-6
MDL number:MFCD00078422
EINECS number:205-183-2
RTECS number:NC4725000
BRN number:3919107
PubChem number:24885293
Physical property data
1. Properties: White or milky white, shiny scaly crystals. The color darkens over time. White or brown scaly crystals.
2. Melting point (℃): 163-164
3. Solubility: Easily soluble in water and ethanol.
Toxicological data
Skin/eye irritation data
Rabbit eye contact: 20mg/24H moderate reaction
Acute toxicity data:
Rat oral LD50: 199mg /kg
Rat abdominal LDLo: 50mg/kg
Mouse intravenous LC50: 180mg/kg
Guinea pig skin contact LD50: >5mg/kg
Oncogenic data:
Oral TDLo in rats: 123 gm/kg/78W-C
Oral TCLo in mice: 437 gm/kg/78W- C
Rat oral TD: 9040 mg/kg/65W-C
Mutagenicity data:
Bacteria – Salmonella typhimurium: 100ug/plate
Bacteria – Escherichia coli: 333ug/plate
Insects – grasshopper: 1pph
Human HeLa cell DNA inhibition test: 2mmol/L
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 56.7
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 112
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical number�Number of stereocenters: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 2
Properties and stability
1. White or cream-colored scaly crystals. Melting point 163-164℃, easily soluble in water and alcohol.
2.Can form with copper, iron, aluminum, titanium, zirconium, vanadium, etc. Insoluble in water, can be dissolved by chloroform, ethyl acetate Complexes extracted with organic solvents.
Storage method
Store in a cool, dry place and seal.
Synthesis method
1. Cool the phenylhydrazine ether solution in an ice melt, and pass in ammonia to excess. While stirring and continuing to pass ammonia, add n-butyl nitrite dropwise, finish the addition in about 1 hour, and stir for another 15 minutes. Filter out the cuproteline precipitate, wash with ether, and dry to obtain the finished product. This product should be stored in a brown glass bottle, with a small amount of ammonium carbonate wrapped in paper (or cloth) placed in the bottle as a stabilizer
2.Cool the phenylhydrazine ether solution, pass in excess ammonia, and add n-butyl nitrite dropwise, filter out the copper-iron reagent precipitate, wash with ether, and dry.
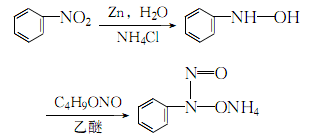
3.Mix nitrobenzene, ammonium chloride and water, heat to 40℃, add the theoretical amount of For more than 85% zinc powder, control the temperature to 60-65°C. After adding, react for half an hour and let it stand. Separate the supernatant and add it to the ice-salt mixture, cool and crystallize. Dissolve the filtered crystalline β-phenylhydroxylamine with diethyl ether, filter out the insoluble matter, cool to 0°C, quickly pass in excess ammonia, and then , while continuing to circulate ammonia, add newly distilled n-butyl nitrite (95g of ester added per 100g of phenylhydrazone), and control the ester addition rate to control the reaction temperature not to exceed 10°C. After the reaction is completed, continue stirring for more than 10 minutes, let it stand, filter, wash the crystals with diethyl ether several times, lay them flat and dry naturally to obtain the finished product.
The process reaction is:
Purpose
1. Chemical analysis reagents for determination of aluminum, copper, iron, titanium, zirconium and other metals.
2.It is used to separate copper and iron from other metal ions; to determine iron by precipitation from strong acid solution; and to determine aluminum colorimetrically. It is also used for the determination of bismuth, gallium, mercury, manganese, niobium, tin, tantalum, thorium, zirconium and other elements.
3. Used as a precipitating agent to precipitate Al3+, Cu2+ Fe3+ , Hg2+ , Sn2+, Ti4+ plasma. Used for precipitation separation or weighing analysis. The precipitate is soluble in organic solvents and therefore can be used as an extraction agent for extraction separation or photometric analysis.

 微信扫一扫打赏
微信扫一扫打赏

