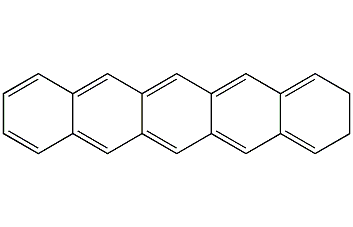
Structural formula
| Business number | 03Q4 |
|---|---|
| Molecular formula | C22H14 |
| Molecular weight | 278.35 |
| label |
2,3,6,7-Dibenzoanthracene, aromatic compounds |
Numbering system
CAS number:135-48-8
MDL number:MFCD00003710
EINECS number:205-193-7
RTECS number:None
BRN number:1912418
PubChem number:24869414
Physical property data
None
Toxicological data
None
Ecological data
None
Molecular structure data
Molecular property data: 1、 Molar refractive index90.98 2、 Molar volume(m3/mol):228.1 3、 Isotonic specific volume(90.2K):610.1 4、 Surface tension(3.0 dyne/cm):51.1 5、 Polarizability0.5 10-24 cm3):36.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 325
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Dark blue needle-shaped crystals with purple luster. Sensitive to light and air. After decompression in carbon dioxide gas flow, it begins to sublimate at approximately 300°C and decomposes in air below 300°C. Slightly soluble in organic solvents, almost soluble in water. Boiling point 290~300℃ (sublimation).
Storage method
Store in argon-filled seal.
Synthesis method
None
Purpose
Used in organic synthesis.
6
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 325
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Dark blue needle-shaped crystals with purple luster. Sensitive to light and air. After decompression in carbon dioxide gas flow, it begins to sublimate at approximately 300°C and decomposes in air below 300°C. Slightly soluble in organic solvents, almost soluble in water. Boiling point 290~300℃ (sublimation).
Storage method
Store in argon-filled seal.
Synthesis method
None
Purpose
Used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

