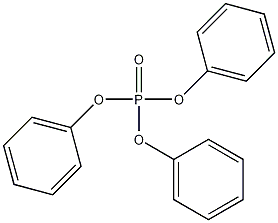
Structural formula
| Business number | 038A |
|---|---|
| Molecular formula | C18H15O4P |
| Molecular weight | 326.28 |
| label |
Triphenyl phosphate (TPP), triphenyl phosphate, Triphenyl phosphate (TPP), Phosphoric acid phenyl ester, flame retardant plasticizer, fire resistant solvents, plastic plasticizer, Impregnation of roof paper |
Numbering system
CAS number:115-86-6
MDL number:MFCD00003031
EINECS number:204-112-2
RTECS number:TC8400000
BRN number:1888236
PubChem number:24889786
Physical property data
1. Properties: White, odorless crystalline powder, slightly deliquescent.
2. Density (g/mL, 50/4℃): 1.205
3. Relative vapor density (g/mL, air=1): 9.4
4. Melting point (ºC): 49~51
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 1.47KPa): 245
7. Refractive index (60ºC): 1.552
8. Flash point (ºC, closed): 220
9. Flash point (ºC, open): 235
10. Viscosity (mPa·s, 50ºC): 11
11. Vapor pressure (mmHg, 200ºC): 1.3
12. Saturated vapor pressure (kPa, 20ºC): 0.01
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Not determined
18. The lower explosion limit (%, V/V) is not determined:
19. Solubility: Insoluble in water, can be mixed with alcohol, ether, chloroform, Acetone, benzene, etc. are miscible. Soluble in most paint solvents, thinners and oils.
Toxicological data
1. Acute toxicity: Oral LD50 in mice: 1300 mg/kg; Oral LD50 in rats: 3000 mg/kg
Rabbit transdermal LD50: 3000 mg/kg; Cat subcutaneous injection LD50: 0.1 ~0.2mL/kg.
2. It is not a skin irritant and is not easily absorbed by the skin. Enters animals and causes flaccid paralysis. The maximum allowable concentration in the workplace is 3 mg/m3 (USA).
Ecological data
Slightly harmful to water.
Molecular structure data
None
�Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 44.8
7. Number of heavy atoms: 23
8. Surface charge: 0
9. Complexity: 325
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It does not decompose at room temperature and pressure, is miscible with ether, chloroform, acetone, etc., is slightly soluble in ethanol, insoluble in water, and has flame retardancy. Solubility in water at 34°C is 0.001%. Highly toxic.
Chemical properties: Triphenyl phosphate is relatively stable to acids and prone to saponification reactions to alkali. When boiled with barium hydroxide alcohol solution, diphenyl barium phosphate is generated. Nitration with nitric acid (relative density 1.5) under cooling mainly produces tris(4-nitrophenyl)phosphate. When nitration is carried out with a mixed acid of nitric acid and sulfuric acid, tris(2,4-dinitrophenyl)phosphate is produced. Triphenyl phosphate generates phenol and a small amount of diphenyl ether and xanthone when heated together with potassium carbonate. When heated with calcium oxide, aluminum oxide or zinc oxide, phenol and phenylanthracene are generated. Treatment with magnesium oxide produces a small amount of diphenyl ether. Triphenyl phosphate is treated with sodium ethoxide to form the sodium salt of diethyl phosphate and ethyl phenyl ether.
Storage method
Sealed and stored in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with corresponding varieties and quantities of consumables. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Methods include: (1) Phosphorus oxychloride direct method (hot method) and phosphorus trichloride indirect method (cold method). (1) Thermal method Dissolve phenol in pyridine and anhydrous benzene solvent, slowly add phosphorus oxychloride at a temperature not exceeding 10°C, and then react at reflux humidity for 3-4 hours. After cooling to room temperature, the reactant is Wash with water to recover pyridine, use it to dry sodium sulfate for dehydration, filter to remove sodium sulfate, and distill under normal pressure to recover benzene; distill under reduced pressure to collect the 243-245°C/1.47KPa fraction, and obtain the finished product after cooling, crystallization, and crushing. 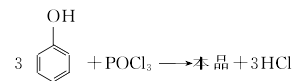 (2) Cold method Dissolve phenol in pyridine and anhydrous benzene solvent, and then heat it at 40℃ Add phosphorus trichloride dropwise to generate triphenyl phosphite, continue to introduce chlorine gas at 70°C to generate triphenyl dichlorophosphoric acid, and then hydrolyze it at 80°C to generate triphenyl phosphate. The hydrolyzate is washed with water, neutralized with alkali, concentrated and then distilled under reduced pressure to obtain the finished product. Refining method: often contains impurities such as phenol, phosphoric acid and acidic phenyl phosphate. Use ethanol or a mixture of ethanol and solvent gasoline for recrystallization and refinement.
(2) Cold method Dissolve phenol in pyridine and anhydrous benzene solvent, and then heat it at 40℃ Add phosphorus trichloride dropwise to generate triphenyl phosphite, continue to introduce chlorine gas at 70°C to generate triphenyl dichlorophosphoric acid, and then hydrolyze it at 80°C to generate triphenyl phosphate. The hydrolyzate is washed with water, neutralized with alkali, concentrated and then distilled under reduced pressure to obtain the finished product. Refining method: often contains impurities such as phenol, phosphoric acid and acidic phenyl phosphate. Use ethanol or a mixture of ethanol and solvent gasoline for recrystallization and refinement. 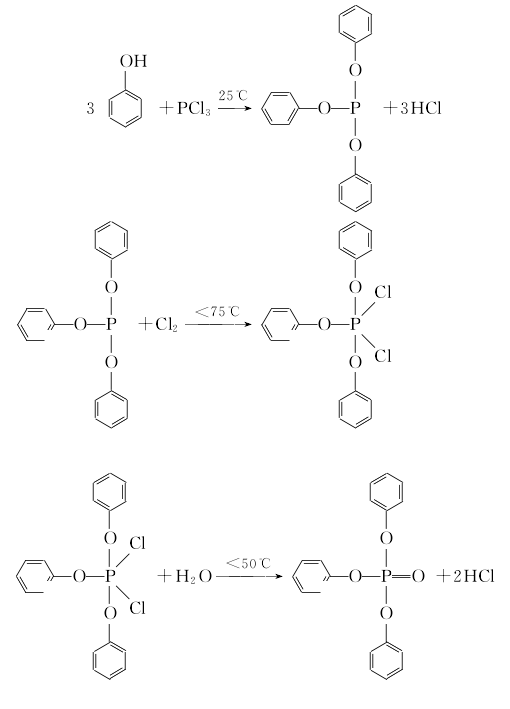
Purpose
1. Used as sizing agent for nitrocellulose and cellulose acetate, flame retardant plasticizer, fire-resistant solvent, nitrocellulose paint, synthetic resin, roof paper, and camphor substitute in celluloid manufacturing.
2.Flame retardant plasticizer. It is mainly used as a flame retardant plasticizer for engineering plastics and phenolic resin laminated boards. It is also used as a flame retardant plasticizer for nitrocellulose and cellulose acetate (film base) and a plasticizer for polyvinyl chloride. It is also used as a softener for synthetic rubber, a gasoline-resistant agent, a non-flammable substitute for camphor in viscose fibers, and a raw material for the manufacture of trimethyl phosphate, etc.

 微信扫一扫打赏
微信扫一扫打赏

