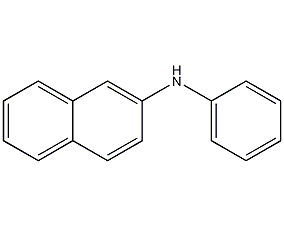
Structural formula
| Business number | 03QM |
|---|---|
| Molecular formula | C16H13N |
| Molecular weight | 219.28 |
| label |
2-(N-anilino)naphthalene, Anti-aging agent, Neozon D, 2-(N-phenylamino)naphthalene, Antioxidant D, Antioxidant D, Antonio cases D, N-Phenyl-β-naphthylamine, 2-Anilinonaphthalene, Universal antioxidant |
Numbering system
CAS number:135-88-6
MDL number:MFCD00004052
EINECS number:205-223-9
RTECS number:QM4550000
BRN number:2211188
PubChem number:24850714
Physical property data
1. Properties: light gray needle-like crystals or powder with ammonia smell. [1]
2. Melting point (℃): 108[2]
3. Boiling point (℃): 395.5 [3]
4. Relative density (water = 1): 1.23[4]
5. Octanol/ Water partition coefficient: 4.38[5]
6. Solubility: Insoluble in water, soluble in ethanol, acetone, and benzene. [6]
Toxicological data
1. Acute toxicity[7]
LD50: 8730mg/kg (rat oral); 1450mg/kg (mouse oral Oral)
LC50: 1920mg/m3 (rat inhalation, 4h)
2. Irritation No data available yet
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[8] Japanese MITI test, The initial concentration is 100ppm, and the degradation is 0~29% after 14 days.
3. Non-biodegradability[9] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 55 minutes (theoretical).
4. Bioconcentration [10] BCF: 147 (fathead minnow, 25℃, contact time 32d).
Molecular structure data
1. Molar refractive index: 73.46
2. Molar volume (cm3/mol): 189.5
3. Isotonic specific volume (90.2K ): 504.4
4. Surface tension (3.0 dyne/cm): 50.1
5. Polarizability (0.5 10-24cm 3): 29.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 12
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 232
10. Number of isotope atoms: 0
11. Determine the atomic positionNumber of ��centers: 0
12. Number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Combustible, low toxicity, easily oxidized and discolored when exposed to air and sunlight.
2. Dilute hydrochloric acid reacts with heat to produce a green reaction. With nitric acid, nitrogen dioxide and sodium sulfite, it produces grape red and muddy red.
3. Stability[11] Stable
4. Incompatible substances[12] Strong oxidants, strong acids
5. Polymerization hazard[13] No polymerization
Storage method
Storage Precautions[14] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is obtained by the condensation of 2-naphthol and aniline at 250°C under the catalysis of aniline hydrochloride. Add 2-naphthol and benzene into the condensation pot to melt, and add aniline hydrochloride for condensation. After the reaction is completed, neutralize with sodium carbonate. Excess aniline is steamed out, and then dehydrated and dried under vacuum. After slicing, powdering, and packaging, the anti-aging agent is obtained. Raw material consumption (kg/t) 2-naphthol (98.5%) 660 Aniline (99.2%) 425
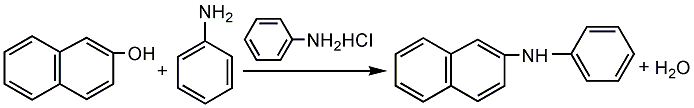
Put aniline and naphthol into the reaction kettle at a mass ratio of 4.28:6.63. After heating and melting, stir Continue to heat to above 132°C, then add 0.09 parts of aniline hydrochloride as a catalyst, raise the temperature to 170°C, and react for 4 hours. Then raise the temperature to 250°C, continue the heating reaction until the 2-naphthol content is below 1%, add soda ash to neutralize the aniline hydrochloride to neutrality, conduct vacuum distillation, and recover aniline until the distillation temperature reaches 235~240°C When, take a sample to analyze the content of aniline in the kettle liquid. When the newly prepared 10% bleaching powder saturated solution is added dropwise to the sample solution and no purple reaction occurs, the distillation can be stopped, and the finished product can be obtained by discharging, drying and crushing while hot.
Purpose
1. This product is a general-purpose antioxidant for natural rubber, diene synthetic rubber, chloroprene rubber and base latex. It has good protection against heat, oxygen, flexing and general aging, and is slightly better than antioxidant A. It has an inhibitory effect on harmful metals, but is weaker than antioxidant A. If used together with antioxidant 4010 or 4010NA, the resistance to heat, oxygen, flex cracking, and ozone will be significantly improved. This product has little effect on the vulcanization speed of natural rubber, nitrile rubber, and styrene-butadiene rubber, but has a slight delay effect on chloroprene rubber. This product is easily decomposed in dry glue and easily dispersed in water. The solubility of this product in rubber is about 1.5%. If the dosage does not exceed 1 part, blooming will not occur. This product is polluting and will gradually turn gray-black under sunlight, so it is not suitable for white or light-colored products. It is mainly used to manufacture industrial products such as tires, hoses, tapes, rubber rollers, rubber shoes, and wire and cable insulation layers. Anti-aging agent Butyl can also be used as a stabilizer during post-processing and storage of various synthetic rubbers, and can be used as a heat-resistant anti-aging agent for polyformaldehyde. This product has certain irritation to the skin.
2. Used as an anti-aging agent for natural rubber and synthetic rubber. It has protective effect against aging caused by oxygen and heat flexing, and has a certain inhibitory effect on harmful metals. The reference dosage is 1 to 2 parts by mass. It is polluting and will discolor the rubber. Anti-aging agent D can also be used as a stabilizer during post-processing and storage of various synthetic rubbers. Because of its high toxicity, it should be used in limited quantities.
3. Used as rubber antioxidant, lubricant, and polymerization inhibitor. [15]

 微信扫一扫打赏
微信扫一扫打赏

