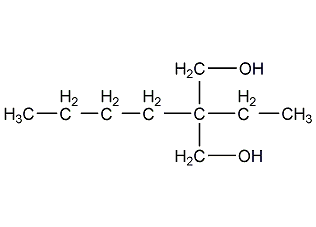
Structural formula
| Business number | 038D |
|---|---|
| Molecular formula | C6H12Cl3O4P |
| Molecular weight | 285.49 |
| label |
Tris(beta-chloroethyl phosphate), trichloroethyl phosphate, Trichloroethyl phosphate (TCEP), Tris(2-chloroethyl)phosphate, Phosphate (β-chloro-ethyl), Phosphate trichloro-ethyl, Phosphate trichloro-ethyl ester, TCEP, flame retardant, plasticizer, Rare metal extractants, Modifier |
Numbering system
CAS number:115-96-8
MDL number:MFCD00000967
EINECS number:204-118-5
RTECS number:KK2450000
BRN number:None
PubChem number:24847295
Physical property data
1. Properties: Light yellow oily liquid with light creamy smell.
2. Density (g/mL, 20/4℃): 1.390
3. Melting point (ºC): -64
4 . Boiling point (ºC, 101.3KPa): 330
5. Boiling point (ºC, 1.3KPa): 194
6. Refractive index (20ºC): 1.4731
7. Flash point (ºC): 232
8. Autoignition point or ignition temperature (ºC): 601.6
9. Vapor pressure (mmHg, 25ºC): <10
10. Vapor pressure (kPa, 145ºC): 0.067
11 Viscosity (mPa·s): 0.038-0.047
12. Solubility: soluble in alcohol , ketones, esters, ethers, benzene, toluene, xylene, easily soluble in chloroform and carbon tetrachloride.
Toxicological data
1. Acute toxicity: Oral – rat LD50: 1230 mg/kg; Oral – mouse LC50: 1866 mg/kg
2. Irritation data: Skin – rabbit 10 mg/24 hours Mild Degree; Eyes – Rabbit 500 mg/24 hours Mild
Ecological data
Slightly harmful to water.
Molecular structure data
1. Molar refractive index: 56.50
2. Molar volume (cm3/mol): 204.9
3. Isotonic specific volume (90.2K ): 517.2
4. Surface tension (dyne/cm): 40.5
5. Polarizability (10-24cm3): 22.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.3
2. Hydrogen bond supply�Number: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 9
5. Number of tautomers :None
6. Topological molecule polar surface area 44.8
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 152
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain atoms Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. It does not decompose under normal temperature and pressure. Avoid contact with strong oxidants, strong acids and strong alkali. 2. Soluble in alcohol, ketone, ester, ether, benzene, toluene, xylene, chloroform, carbon tetrachloride, slightly soluble in water, insoluble in aliphatic hydrocarbons, solubility in water (20°C) 4.64%. It has good compatibility with polymers such as cellulose acetate, cellulose nitrate, butyl cellulose acetate, ethyl cellulose, polyvinyl chloride, polystyrene, phenolic resin, etc. A small amount of decomposition occurs in sodium hydroxide solutions. It has excellent flame retardancy, excellent low temperature resistance and UV resistance. Its vapor can only be burned when directly ignited by an open flame above 225℃, but it will self-extinguish immediately if the fire source is removed. Using this product as a flame retardant can not only improve the material grade of the flame retardant material, but also improve the water resistance, acid resistance, cold resistance and antistatic properties of the flame retardant material. Poisoned.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. 2. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. 3. Equip with corresponding varieties and quantities of fire-fighting equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
There are three industrial preparation methods: the reaction of phosphorus oxychloride and chloroethanol; the reaction of phosphorus oxychloride and chloroethanol and then oxidation; the reaction of phosphorus oxychloride and ethylene oxide (common industrial method). 1. Phosphorus oxychloride and ethylene oxide react with sodium metavanadate as a catalyst at 50°C. The reactants are neutralized, washed with water, and vacuum dehydrated to remove low boilers to obtain the finished product. Chloroethanol can also be used as raw material and reacted with phosphorus oxychloride or phosphorus trichloride to produce tris(2-chloroethyl)phosphate. 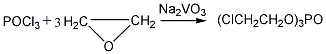
2. Put 326 kg of phosphorus oxychloride and 1.0 kg of sodium metavanadate into the reaction In the cauldron. Fill the reaction kettle with nitrogen to drive out the air, introduce 650 kg of ethylene oxide under vacuum, and stir at 45 to 50°C for 2 to 3 hours. After evaporating excess ethylene oxide, add alkali to neutralize to neutrality, wash with water, and dehydrate in vacuum. Get the finished product. The reaction formula is as follows:
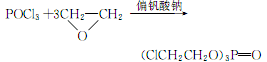
3.Put phosphorus oxychloride and sodium metaphosphate into the reactor. Fill with nitrogen to drive out the air, introduce ethylene oxide under vacuum, and stir at 45 to 50°C for 2 to 3 hours. After evaporating excess ethylene oxide, add alkali to neutralize to neutrality, wash with water, and vacuum dehydrate to obtain the finished product.
Purpose
1. This product has excellent flame retardancy, excellent low temperature resistance and ultraviolet resistance. Its vapor can only be burned when directly ignited with an open flame above 225℃, but it will self-extinguish immediately if the fire source is removed. . Using this product as a flame retardant can not only improve the material grade of the flame retardant material, but also improve the water resistance, acid resistance, cold resistance and antistatic properties of the flame retardant material. It is often used in flame-retardant paints and coatings based on nitrocellulose and acetate fiber, unsaturated polyester, polyurethane, acrylate, phenolic resin, etc. It can also be used as a plasticizing flame retardant for soft polyvinyl chloride. The addition amount of this product is 10% to 20% for unsaturated polyester, about 10% for rigid polyurethane foam (with flame-retardant polyether as raw material), and is used as auxiliary plasticizer for soft polyvinyl chloride. When using flame retardants, it is 5% to 10%. Flame retardant, separation solvent or extraction agent for uranium, thorium, plutonium, technetium and other rare metals.
2.This product is widely used as a flame retardant in chemical fiber fabrics and cellulose acetate. In addition to being self-extinguishing, it can also improve water resistance. , cold resistance and antistatic properties. The general dosage is 5 to 10 servings. This product is an excellent flame retardant for synthetic materials and has a good form-increasing effect. It is widely used in cellulose acetate, nitrocellulose varnish, ethyl cellulose, polyvinyl chloride, polyvinyl acetate, polyurethane, phenolic resin, etc. In addition to being self-extinguishing, the produced product can also improve the physical properties of the product, making the product soft to the touch. It can also be called an extractant for petroleum additives and rare elements, and is also the main flame retardant material for flame retardant rubber conveyor belts. , the general addition amount is 5% to 10%.
3.Used as additive halogenated phosphate flame retardants and plasticizers. The molecule contains both phosphorus and chlorine, which has significant flame retardant effect, is not easy to volatilize and hydrolyze, and has good stability against ultraviolet rays. Suitable for phenolic resin, polyvinyl chloride, polyvinyl acetate, polyurethane, etc. It is also used as a flame retardant for nitrocellulose coatings, a flame retardant plasticizer for polyvinyl chloride, a metal extractant, a gasoline additive and a polyimide processing aid. Can improve water resistance, weather resistance, cold resistance, and antistatic properties. The reference dosage is 5% to 20%.

 微信扫一扫打赏
微信扫一扫打赏

