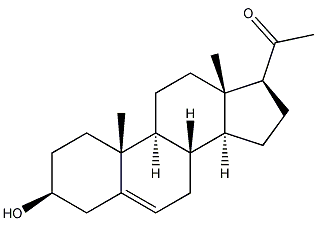
structural formula
| business number | 03wq |
|---|---|
| molecular formula | c21h32o2 |
| molecular weight | 316.48 |
| label |
3-hydroxy-5-pregnenone, 5-pregnene-3β-ol-20-one, 3β-hydroxypregnant-5-en-20-one, 3β-hydroxy-5-pregnen-20-one |
numbering system
cas number:145-13-1
mdl number:mfcd00003628
einecs number:205-647-4
rtecs number:tu5560700
brn number:none
pubchem number:24899008
physical property data
1. physical property data:
1. character:white crystalline powder
2. melting point (ºc): 188-190ºc
3. specific rotation (º):28.5 º
4. dissolve sex: insoluble in water.
toxicological data
2. toxicological data:
1, acute toxicity: mouse abdominal cavityld: >200 mg/kg.
ecological data
3. ecological data:
usually for water is not hazardous and materials should not be discharged into the surrounding environment without government permission.
molecular structure data
none
compute chemical data
4. calculated chemical data:
1. hydrophobic parameters calculate reference value (xlogp):4.2
2. hydrogen bonding number of donors: 1
3. hydrogen bonding number of receptors: 2
4. rotatable number of chemical bonds: 1
5. topological molecules polar surface area (tpsa):37.3
6. heavy atoms quantity: 23
7. surface charge :0
8. complexity:550
9. isotope atomic number:0
10. determine the number of atomic stereocenters:7
11. uncertain number of atomic stereocenters:0
12. determine the number of stereocenters of chemical bonds:0
13. uncertain number of chemical bond stereocenters:0
14. number of covalent bond units: 1
properties and stability
stable under normal temperature and pressure.
storage method
seal and store in a dry and cool place.
synthesis method
heat gestadienolone acetate, ethanol, and reed nickel together, 50℃turn left and right to dissolve everything, then vacuum and pass in hydrogen, repeat 3 after several times, pass hydrogen under stirring, and then add hydrogen to 34-36℃reaction3.5 h. leave to stand, vent the hydrogen gas through nitrogen, and filter out the catalyst. concentrate the filtrate to recover most of the ethanol and precipitate crystals. put the crystals into a hydrolysis pot and evaporate the ethanol under reduced pressure. after cooling, add methanol and heat to reflux to dissolve all. add 50% potassium carbonate aqueous solution and reflux 1.5h, concentrate under reduced pressure to recover methanol, and cool to 5℃the following, filter take the crystals, wash them with warm water until they are neutral, and dry them. then dissolve it in 10 times of ethanol, add activated carbon to decolorize, and recrystallize 1 times to obtain pregnenolone.
purpose
1.for biochemical research. intermediate of hormone drug progesterone.
2.for the synthesis of steroid drug intermediates and steroid drugs.
3.used as an intermediate for pharmaceutical progesterone.
size: 9pt; font-family: verdana; mso-bidi-font-family: tahoma”>
synthesis method
heat gestadienolone acetate, ethanol, and reed nickel together, 50℃turn left and right to dissolve everything, then vacuum and pass in hydrogen, repeat 3 after several times, pass hydrogen under stirring, and then add hydrogen to 34-36℃reaction3.5 h. leave to stand, vent the hydrogen gas through nitrogen, and filter out the catalyst. concentrate the filtrate to recover most of the ethanol and precipitate crystals. put the crystals into a hydrolysis pot and evaporate the ethanol under reduced pressure. after cooling, add methanol and heat to reflux to dissolve all. add 50% potassium carbonate aqueous solution and reflux 1.5h, concentrate under reduced pressure to recover methanol, and cool to 5℃the following, filter take the crystals, wash them with warm water until they are neutral, and dry them. then dissolve it in 10 times of ethanol, add activated carbon to decolorize, and recrystallize 1 times to obtain pregnenolone.
purpose
1.for biochemical research. intermediate of hormone drug progesterone.
2.for the synthesis of steroid drug intermediates and steroid drugs.
3.used as an intermediate for pharmaceutical progesterone.

 微信扫一扫打赏
微信扫一扫打赏

