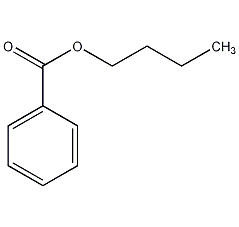
Structural formula
| Business number | 03QZ |
|---|---|
| Molecular formula | C11H14O2 |
| Molecular weight | 178.23 |
| label |
n-butyl benzoate, n-butyl benzoate, 1-Butyl benzoate, Solvent for cellulose esters, plasticizer, raw materials for spices, aromatic compounds |
Numbering system
CAS number:136-60-7
MDL number:MFCD00009439
EINECS number:205-252-7
RTECS number:DG4925000
BRN number:1867073
PubChem number:24901912
Physical property data
1. Properties: colorless oily liquid with slight fruity aroma.
2. Relative density (g/mL, 25/25℃): 1.005
3. Relative density (25℃, 4℃): 0.950286.7
4. Melting point (ºC): -22
5. Boiling point (ºC, normal pressure): 250
6. Relative density (20℃, 4℃): 1.0061
7. Refractive index (25ºC): 1.4940
8. Flash point (ºC, opening): 107
9. Refraction at room temperature Rate (n20): 1.4964
10. Liquid phase standard hot melt (J·mol-1·K-1): 292.4
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18 . Lower explosion limit (%, V/V): Undetermined
19. Solubility: Miscible with ethanol and ether, almost insoluble in water. Dissolved in water at 20°C <0.01%; water dissolved in butyl benzoate 0.32%.
Toxicological data
1. Skin/eye irritation data: rabbit skin contact: moderate reaction at 500mg
2. Acute toxicity data: rat oral LD50: 5100mg/kg; mouse oral LD50: 3450mg/ kg; Rats inhaled saturated vapor for 8 hours without death.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 51.92
2. Molar volume (cm3/mol): 176.8
3. Isotonic specific volume (90.2K ): 431.4
4. Surface tension (3.0 dyne/cm): 35.4
5. Polarizability (0.5 10-24cm 3): 20.58
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 148
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
The chemical properties are relatively stable and hydrolysis occurs under the action of caustic alkali. Non-corrosive to metals.
Storage method
Pay attention to fire sources and store in a cool place. It can be stored in iron, mild steel or aluminum containers.
Synthesis method
Obtained from the esterification of benzoic acid and butanol. Heat benzoic acid, butanol, sulfuric acid and carbon tetrachloride together to reflux for 8 hours. After removing a certain amount of water, remove excess butanol under reduced pressure, then wash until neutral, dry and distill under reduced pressure to obtain the finished product.
Refining method: Contains impurities such as free acid and alcohol. During refining, wash with sodium bicarbonate or sodium carbonate solution, dry with anhydrous potassium carbonate or sodium sulfate and then distill.
Purpose
Used as a solvent for cellulose esters, a plasticizer and a raw material for spices.

 微信扫一扫打赏
微信扫一扫打赏

