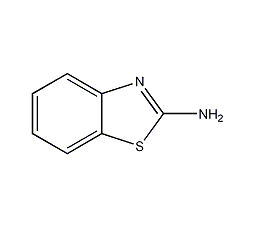
Structural formula
| Business number | 03R4 |
|---|---|
| Molecular formula | C7H6N2S |
| Molecular weight | 150.20 |
| label |
2-Benzothiazolamine, 2-amino-benzothiazole, 2-Phenylthiazolamine, 2-Aminobenzothiazole, 2-Benzothiazolamine, Heterocyclic compounds |
Numbering system
CAS number:136-95-8
MDL number:MFCD00005785
EINECS number:205-268-4
RTECS number:DL1050000
BRN number:116315
PubChem number:24846830
Physical property data
1. Character: White leaf-like crystal
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL , air=1): Not determined
4. Melting point (ºC): 128
5. Boiling point (ºC, normal pressure): Not determined
6 . Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9 . Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility : Easily soluble in ethanol, ether and chloroform, soluble in concentrated acid, very slightly soluble in water.
Toxicological data
Acute toxicity data:
Mouse oral LD50: >1mg/kg
Mouse intraperitoneal LD50: 200mg/kg
Mouse intravenous LD50 : 126mg/kg
Mutation data:
Bacteria – Salmonella typhimurium: 2500ug/plate
Ecological data
None
Molecular structure data
1. Molar refractive index: 44.80
2. Molar volume (cm3/mol): 108.5
3. Isotonic specific volume (90.2K): 314.2
4. Surface tension (3.0 dyne/cm): 70.2
5. Polarizability (0.5 10-24cm3): 17.76
Compute chemical data
1. Hydrophobic parameter calculation��Reference value (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 67.2
7. Number of heavy atoms :10
8. Surface charge: 0
9. Complexity: 129
10. Number of isotope atoms: 0
11 .Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Irritant and harmful if inhaled, taken orally or in contact with skin.
Storage method
Stored sealed and protected from light.
Synthesis method
Obtained from the reaction of phenylthiourea and sulfur chloride. First, suck anhydrous chloroform into a glass-lined pot, add phenylthiourea while stirring, slowly raise the temperature to 60-63°C, steam out the aqueous chloroform, cool to 40°C, add sulfur chloride, and keep the gas phase temperature not higher than 40°C. , to prevent the material from clumping. 4 hours after the addition, the temperature was gradually raised to 61°C, and the reaction was refluxed at 61-64°C for 10 hours. Then drain it into a distillation pot containing a certain amount of water and activated carbon, and use direct steam distillation to recover chloroform until the liquid phase temperature is 98°C and maintain it for 15 minutes. After recovery is completed, add water to dilute, add ice to cool, add liquid alkali to neutralize to pH 4.1, add activated carbon and stir. Cool and suction filtrate, neutralize the filtrate with 30% liquid alkali to a pH value of 7.0-7.6, then cool to 35-40°C, press filter and dry (temperature does not exceed 80°C) to obtain the finished product. The melting point is 128-130°C, and the yield is 74.4%.
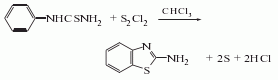
Purpose
1. Organic synthesis. Photographic materials. Preparation of azo dyes.
2. Dye intermediates. It is used to produce cationic violet 3RL, and is also used to synthesize 3-methylbenzothiazole hydrazone to produce cationic violet 2RL, etc. It is also a raw material for other organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

