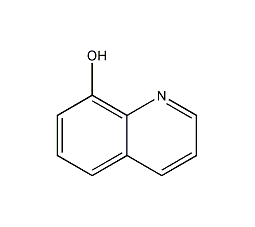
Structural formula
| Business number | 03XE |
|---|---|
| Molecular formula | C9H7NO |
| Molecular weight | 145.16 |
| label |
8-quinoline hydroxide, 8-Hydroxyazepine, Oxyquinoline,Oxychinolin,Oxybenzopyridine,Oxychinoline,Fennosan, 8-Hydroxyquinoline, fungicides, Heterocyclic compounds |
Numbering system
CAS number:148-24-3
MDL number:MFCD00006807
EINECS number:205-711-1
RTECS number:VC4200000
BRN number:114512
PubChem number:24855220
Physical property data
1. Properties: White or light yellow crystal or crystalline powder
2. Melting point (ºC): 75~76
3. Boiling point (ºC): 267
4. Solubility: insoluble in water, ether, Soluble in ethanol, acetone, chloroform, benzene and inorganic acids.
Toxicological data
1. Acute toxicity: Rat mobilize LD50: 1200 mg/kg; mice LC50: 20 gm/kg;
Rat abdominal cavity LD: 43 gm/kg; mice under the skin of the mouse; LCLo: 83600 ug/kg.
2. Inhalation toxicity: Rat: >1210 mg/m3/6H
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 44.06
2. Molar volume (cm3/mol): 115.2
3. Isotonic specific volume (90.2K ): 320.2
4. Surface tension (dyne/cm): 59.7
5. Polarizability (10-24cm3): 17.47
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 33.1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 138
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Storage method
Store sealed in a dry and cool place.
Synthesis method
1. From o-amino groupPhenol is produced through cyclization reaction. Add glycerin to the acid-resistant reaction pot, slowly add concentrated sulfuric acid while stirring, and at the same time add o-aminophenol and o-nitrophenol in sequence, and oleum first adds 65% of the total amount. Heat to 125°C, stop heating, naturally raise the temperature to 140°C, and wait until the temperature drops back to 136°C. Continue to add the remaining oleum, keeping the temperature at 137°C. After adding acid, keep it warm for 4 hours, cool to below 100°C, pump it into an acid-resistant pot containing 10 times the amount of water (calculated as o-aminophenol), stir, heat to 75-80°C, and use 30% sodium hydroxide The solution is neutralized to a pH of 7-7.2. Release the precipitate while it is hot, cool it into a block, and sublime it under reduced pressure to obtain the finished product of 8-hydroxyquinoline.
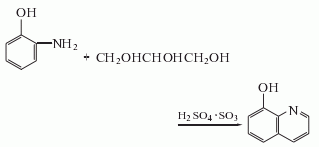
2.Mix o-aminophenol, o-nitrophenol, glycerin, and glacial acetic acid, add concentrated sulfuric acid in portions, and heat the mixture until it begins to boil. Then reflux on an oil bath, and remove unreacted o-nitrophenol by steam distillation. Cool, neutralize with sodium hydroxide solution, and recover by steam distillation to obtain 8-hydroxyquinoline.
3.Sulfonate quinoline to obtain 8-sulfonate quinoline, and then undergo alkali fusion to obtain 8-hydroxyquin The pholine base is finally acidified to obtain 8-hydroxyquinoline. The preparation process is as follows:
① The sulfonation process uses quinoline as raw material. Slowly add the raw material to sulfuric acid under stirring conditions, heat in a sand bath, and sulfonate 2~ 3h. Then it is naturally cooled to room temperature, further cooled with ice water, filtered and dried to obtain the product 8-sulfonate quinoline.
② In the alkali melting process, slowly add the sulfonated product to the molten alkali under stirring conditions, and melt it at about 200-240°C for 30-40 minutes. After cooling, add hot water and heat to dissolve it all. , after cooling to room temperature, neutralize with dilute acid to pH = 7. After crystallization, filter with suction and dry the filter cake.
③ Distillation process: The alkali fusion product is distilled with water vapor, cooled, and crystallized to obtain the product 8-hydroxyquinoline.
Purpose
1. Used as a pharmaceutical intermediate, a raw material for the synthesis of clostriatin, clioquinoline, and paracetamol, as well as an intermediate for dyes and pesticides. This product is an intermediate of halogenated quinoline anti-amoebic drugs, including quiniodoform, clioiodoquinoline, biiodoquinoline, etc. These drugs exert anti-amoebic effects by inhibiting intestinal commensal bacteria and are effective against amoebic dysentery but have no effect on extraintestinal amebic protozoa. In recent years, it has been reported abroad that this type of drug can cause subacute myelooptic neuropathy, so the drug has been banned in Japan and the United States. This disease is less common with iodoquinoline than with clioquinoline. 8-Hydroxyquinoline is also an intermediate for dyes and pesticides. Its sulfate and copper salts are excellent preservatives, disinfectants and fungicides. This product is a complex titration indicator for chemical analysis.
2.Used as complexing agent and extraction agent for precipitation and separation of metal ions, it can interact with Cu2+, Ca2+ , Mg2+ , Ga7+, and other metal ion complexes. Standard for determination of heterocyclic nitrogen by organic microanalysis, Organic Synthesis. It is also an intermediate for dyes, pesticides and halogenated quinoline anti-amoeba drugs. Its sulfates and copper salts are excellent preservatives.
3.Added into epoxy resin adhesive to improve the bonding strength and heat resistance to metal (especially stainless steel) Aging resistance, the dosage is generally 0.5 to 3 parts. It is an intermediate of halogenated quinoline anti-amoebic drugs, as well as an intermediate of pesticides and dyes. It can be used as an antifungal agent, an industrial preservative, a stabilizer for polyester resin, phenolic resin and hydrogen peroxide, and a complex titration indicator for chemical analysis.
4.This product is an intermediate of halogenated quinoline drugs, dyes and pesticides. Its sulfate and copper salts are excellent preservatives, disinfectants and fungicides. The maximum allowable content (mass fraction) in cosmetics is 0.3%. It is prohibited in sunscreen products and products for children under 3 years old (such as talcum powder), and “not allowed for children under 3 years old” should be marked on the product label. When treating bacterially infected skin and bacterially transmitted eczema
, the mass fraction of 8-hydroxyquinoline in the lotion is 0.001% to 0.02%. It is also used as a disinfectant, antiseptic and bactericide and has strong anti-fungal properties. The content (mass fraction) of 8-hydroxyquinoline potassium sulfate used in skin care creams and lotions is 0.05% to 0.5%.

 微信扫一扫打赏
微信扫一扫打赏

