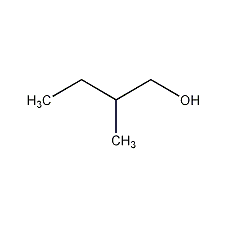
Structural formula
| Business number | 03RJ |
|---|---|
| Molecular formula | C5H12O |
| Molecular weight | 88.15 |
| label |
2-methylbutanol, active amyl alcohol, Optically active pentanol, 2-Methyl-1-butanol, L-2-methylbutanol, DL-sec-Butyl carbinol, Active amyl alcohol, catalyst, alcohol solvent |
Numbering system
CAS number:137-32-6
MDL number:MFCD00004743
EINECS number:205-289-9
RTECS number:EL5250000
BRN number:1718810
PubChem number:24884168
Physical property data
1. Properties: colorless and transparent liquid with special odor.
2. Boiling point (ºC, 101.3kPa): 130
3. Solubility parameter (J·cm-3)0.5
sup>:22.274
4. Relative density (g/mL, 20/4ºC): 0.815
5. Relative density (25℃, 4℃): 0.8150
6. Refractive index (n20ºC): 1.4107
7. Viscosity (mPa·s, 20ºC): 5.09
8. Flash point (ºC, opening): 43
9. Heat of evaporation (KJ/kg, b.p.): 472
10. Vapor pressure (kPa, 65.7ºC): 6.67
11. Solubility (% , water, 20ºC): 2.2
12. Body expansion coefficient (K-1, 20ºC): 0.00078
13. Solubility: slightly soluble in Water is miscible with alcohol and ether.
14. Refractive index at room temperature (n25): 1.4086
15. Critical temperature (ºC): 302.25
16. Critical pressure (MPa): 3.94
17. Eccentricity factor: 0.678
18. van der Waals area (cm2·mol-1 ): 8.970×109
19. van der Waals volume (cm3·mol-1): 62.620
20. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): 3379.8
21. Gas phase standard claimed heat ( Enthalpy) (kJ·mol-1): -302.5
22. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1) : -3325.95
23. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -356.60
24. Liquid phase standard heat Melt (J·mol-1·K-1): 226.7
Toxicological data
1. Skin/eye irritation dataRabbit skin contact: 8193ug/24H mild reaction
2. Acute toxicity data: Rat oral LD50: 1mg/kg; rat abdominal LDLo: 1900mg/kg
Rabbit skin LD50: 3540uL/kg; amphibian-frog parenteral LDLo: 2448mg/kg
3. It is of low toxicity. It has a strong irritating effect on the eyes and a weak irritating effect on the skin, but it can be absorbed by the skin.
Ecological data
None
Molecular structure data
1. Molar refractive index: 26.70
2. Molar volume (cm3/mol): 108.9
3. Isotonic specific volume (90.2K): 245.1
4. Surface tension (3.0 dyne/cm): 25.6
5. Polarizability (0.5 10-24cm3): 10.58
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 27.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Found in burley tobacco leaves.
Storage method
None
Synthesis method
1. It exists in fusel oil and has left-handedness. High-purity dl-2-methylbutanol can be separated from fusel oil using a high-efficiency fractionation tower. To remove the water in 2-methylbutanol, calcium oxide can be refluxed and then fractionated, and the distillate can be refluxed with magnesium and then fractionated.
2. Tobacco: BU, 56; FC, 40.
Purpose
This product is a useful synthetic raw material. In addition to being used as a solvent, it is also used to introduce rotary pentyl groups in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

