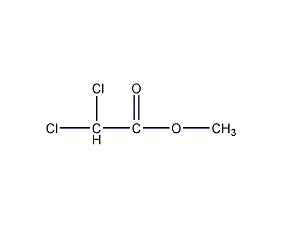
Structural formula
| Business number | 038R |
|---|---|
| Molecular formula | C3H4Cl2O2 |
| Molecular weight | 142.97 |
| label |
Pesticides; aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:116-54-1
MDL number:MFCD00000843
EINECS number:204-146-8
RTECS number:AG6625000
BRN number:1747542
PubChem number:24862526
Physical property data
1. Properties: colorless liquid with ether-like odor. [1]
2. Melting point (℃): -52[2]
3. Boiling point (℃): 143[3]
4. Relative density (water = 1): 1.38[4]
5. Relative vapor Density (air=1): 4.93[5]
6. Saturated vapor pressure (kPa): 0.665 (26.7℃)[6]
7. Octanol/water partition coefficient: 0.81[7]
8. Flash point (℃): 80[8]
9. Solubility: Slightly soluble in water, soluble in ethanol and ether. [9]
10. Relative density (25℃, 4℃): 1.3712
11. Refractive index at room temperature (n20): 1.4426
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
3. Others[10] LCLo: 2000ppm (rat inhalation, 30min)
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 27.37
2. Molar volume (cm3/mol): 104.6
3. Isotonic specific volume (90.2K ): 250.5
4. Surface tension (dyne/cm): 32.7
5. Polarizability (10-24cm3): 10.85
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 71.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[11] Stable
2. Incompatible substances[12] Acids, alkalis, strong oxidants, strong reducing agents, water
3. Conditions to avoid contact[13] Heat, moisture Air
4. Polymerization hazard[14] Does not polymerize
5. Decomposition products[15 ] Hydrogen chloride, phosgene
Storage method
Storage Precautions[16] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the esterification of dichloroacetic acid and methanol. First, put dichloroacetic acid into the reaction pot, heat it to 120°C, add methanol dropwise to react, and the generated ester and water will continue to steam out. The steam condenser will cool it into a mixture of ester, water, alcohol, and acid, and flow into the water separator. The lower part flows into the crude methyl dichloroacetate storage tank through the liquid seal tube, and the alcohol and water overflow from the water separator and return to the reaction pot to continue the reaction. The crude methyl dichloroacetate was neutralized to slightly alkaline with 10% sodium carbonate solution at room temperature, left to stand and separated, and then the ester layer was washed with water. Distill the crude ester under reduced pressure, separate out low-boiling substances such as water and methanol, and collect the fractions above 65°C (8.0kPa) as the finished product. Each ton of product consumes 1100kg of dichloroacetic acid (≥90%) and 360kg of methanol.
![]()
Purpose
1. Intermediates for pharmaceutical synthesis. Used to make drugs such as synmycotoxin and chloramphenicol. Also used in the dye industry.
2. Used as an intermediate in organic synthesis. [17]

 微信扫一扫打赏
微信扫一扫打赏

