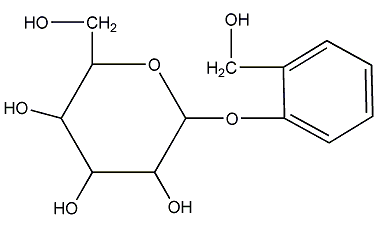
Structural formula
| Business number | 03S0 |
|---|---|
| Molecular formula | C13H18O7 |
| Molecular weight | 286.28 |
| label |
aromatic compounds |
Numbering system
CAS number:138-52-3
MDL number:MFCD00006590
EINECS number:205-331-6
RTECS number:LZ5901700
BRN number:89593
PubChem number:24899442
Physical property data
Properties: colorless crystal or white powder. Hygroscopic.
Relative density: 1.434
Melting point: 199~202
Solubility: Soluble in water, boiling water, ethanol, pyridine, alkali and glacial acetic acid, almost insoluble in ether and chloroform.
Toxicological data
Acute toxicity data :
Mouse abdominal cavity LD:>500mg/kg
Ecological data
None
Molecular structure data
Molecular property data: 1, Molar refractive index:68.41 2, Molar volume(m3/mol):190.3 3, Isotonic specific volume(90.2K):550.7 4, Surface tension(3.0 dyne/ cm):70.1 5, Polarizability(0.5 10 -24cm3):27.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 5
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 120
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 300
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 5
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Store sealed and dry.
Synthesis method
None
Purpose
Naturally found in Salicaceae plant Salix(Salix babylonica L.)in branches, stems and other plants. It can be obtained through chemical synthesis. After oral administration15~30min, salicinogen, salicylic acid, etc. can appear in urine, therefore, it has Antipyretic, analgesic, anti-inflammatory, anti-rheumatic and other effects. Because this transformation is not constant, its therapeutic value is lower than that of salicylic acid. In addition, it also has a bitter taste, stomachic and local anesthetic effects. It can also be used as a biochemical reagent.
Substrate for determination of b-glycosidase .
AN style=”FONT-SIZE: 9pt; FONT-FAMILY: Arial; mso-font-kerning: 0pt; mso-fareast-font-family: Arial”>(0.5 10-24cm3):27.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 5
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 120
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 300
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 5
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Store sealed and dry.
Synthesis method
None
Purpose
Naturally found in Salicaceae plant Salix(Salix babylonica L.)in branches, stems and other plants. It can be obtained through chemical synthesis. After oral administration15~30min, salicinogen, salicylic acid, etc. can appear in urine, therefore, it has Antipyretic, analgesic, anti-inflammatory, anti-rheumatic and other effects. Because this transformation is not constant, its therapeutic value is lower than that of salicylic acid. In addition, it also has a bitter taste, stomachic and local anesthetic effects. It can also be used as a biochemical reagent.
Substrate for determination of b-glycosidase .

 微信扫一扫打赏
微信扫一扫打赏

