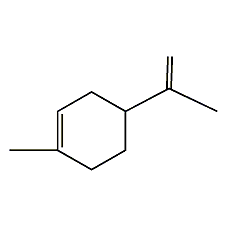
Structural formula
| Business number | 03S5 |
|---|---|
| Molecular formula | C10H16 |
| Molecular weight | 136.23 |
| label |
1,8-terpene, dipentene, Limonene, dipentene, synthetic fragrances, spices and essences, paint solvents, Dispersant, thinner, Enamel paint wetting agent, Rubber desulfurizer |
Numbering system
CAS number:138-86-3
MDL number:MFCD00062992
EINECS number:205-341-0
RTECS number:OS8100000
BRN number:3195091
PubChem number:24902101
Physical property data
1. Properties: colorless liquid with lemon-like aroma. [1]
2. Melting point (℃): -95.5[2]
3. Boiling point (℃): 175.5~176.5[3]
4. Relative density (water=1): 0.842[4]
5. Relative vapor density (air=1): 4.66[5]
6. Saturated vapor pressure (kPa): 0.13 (14℃)[6]
7. Heat of combustion (kJ/mol): -6184.8[7]
8. Octanol/water partition coefficient: 4.57 [8]
9. Flash point (℃): 45 (CC) [9]
10. Ignition temperature (℃) ): 237[10]
11. Explosion upper limit (%): 6.1[11]
12. Explosion lower limit (%): 0.7[12]
13. Solubility: Insoluble in water, miscible in ethanol and ether. [13]
Toxicological data
1. Acute toxicity[14]
LD50: 5300mg/kg (rat oral); 5550μl (4662mg)/kg ( Mouse oral)
LC50: 67500mg/m3 (mouse inhalation)
2. Irritation[15] Rabbit transdermal: 500mg (24h), moderate irritation.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[16] MITI-I test , the initial concentration is 100ppm, the sludge concentration is 30ppm, and the degradation is 41%~98% after 14 days.
3. Non-biodegradability No information available
4. Other harmful effects[17] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 45.35
2. Molar volume (cm3/mol): 163.2
3. Isotonic specific volume (90.2K ): 368.2
4. Surface tension (3.0 dyne/cm): 25.8
5. Polarizability (0.5 10-24cm 3): 17.98
Compute chemical data
1.Reference value for hydrophobic parameter calculation (XlogP): 3.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4 .Number of rotatable chemical bonds: 1
5.Number of tautomers: None
6.Topological molecule polar surface area 0
7.Heavy atoms Number: 10
8. Surface charge: 0
9. Complexity: 163
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[18] Stable
2. Incompatible substances[19] Strong oxidants, strong acids, halogenated hydrocarbons, halogens
3. Conditions to avoid contact[20] Humid air, heat p>
4. Aggregation hazards[21] Aggregation
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants and acids, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Widely found in natural plant essential oils. Among them, the main ones containing dextrorotary body are mandarin oil, lemon oil, orange oil, camphor white oil, etc. Those containing L-body include peppermint oil and so on. Those containing racemates include neroli oil, fir oil and camphor white oil. When manufacturing this product, it is prepared by fractional distillation of the above-mentioned essential oils. Terpenes can also be extracted from general essential oils, or can be obtained as by-products during the processing of camphor oil and synthetic camphor. The obtained dipentene can be purified by distillation to obtain limonene. Use turpentine as raw material, conduct fractional distillation, cut α-pinene, prepare camphene through isomerization, and then fractionate to obtain it. A by-product of camphene is dipentene. In addition, when hydrating turpentine to produce terpineol, dipentene can also be produced as a by-product.
2. Calcium chloride or anhydrous sodium sulfate is dehydrated and then distilled under reduced pressure.
Purpose
1. Used as enamel, fake paint and various oleoresins, resin waxes, metal driers and solvents; used in the manufacture of synthetic resins and synthetic rubber; used in blending orange blossom essence, citrus oil essence, etc.; also Can be made into a substitute for lemon essential oils. Limonene is directionally oxidized to generate carvone; in the presence of inorganic acid, limonene reacts with water to generate α-terpineol and hydrated terpene diol; hydrogenation under the action of platinum or color catalysts generates para-alkanes, and dehydrogenation generates para-alkanes. Flower hydrocarbons. Also used as oil dispersant, rubber additive, wetting agent, etc.
2. Use paint solvent to prevent skinning, gelation and initial hardening of the paint film. Due to its moderate volatility, it has good ductility and leveling properties. Its solubility is similar to turpentine, and it is used as a substitute for turpentine or petroleum solvents in floor waxes and furniture polishes. In addition, it can be used as a dispersant for grease, resin, and pigments, a diluent for synthetic resin varnishes and enamels, a wetting agent for enamel pigments, and a rubber desulfurizer. A mixed solvent composed of petroleum diluent and 1,8-terpene (12% to 20%) can dissolve resinous substances that are insoluble in petroleum.
3. Used as solvent and in organic synthesis. [23]

 微信扫一扫打赏
微信扫一扫打赏

