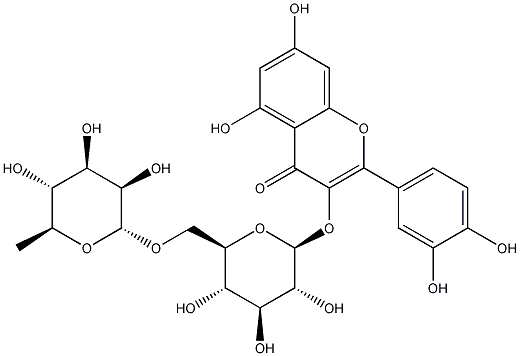
Structural formula
| Business number | 03YY |
|---|---|
| Molecular formula | C27H30O16 |
| Molecular weight | 610.52 |
| label |
Rutin rutin, Luding, Luding powder, Vitamin P, Rutin, 3,3’,4’,5,7-Pentahydroxy-flavon3-(o-rhamnosylglucoside), 3-[[6-O-(-Deoxy-L-mannopyranosyl)-D-glucopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one, Quercetin-3-rutinoside, Consume antioxidants and nutritional enhancers |
Numbering system
CAS number:153-18-4
MDL number:None
EINECS number:205-814-1
RTECS number:VM2975000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: Rutin is a flavonol glycoside widely present in plants. The two glycosides are glucose and rhamnose. It is light yellow or light green needle-like crystals or crystalline powder
2. Melting point (℃): 214~215
3. Specific optical rotation (º, in ethanol): 13.82
4. Solubility: soluble in pyridine, formyl and alkali, slightly soluble in ethanol, acetone and ethyl acetate, almost insoluble in water, chloroform, ether, benzene, carbon disulfide and petroleum ether.
Toxicological data
1. Acute toxicity: rat abdominal LD50: 2 gm/kg; mouse abdominal LC50: 200 mg/kg; mouse intravenous LC50: 950 mg/kg
2. Chronic toxicity/carcinogenesis Properties: Rat oral TDLo: 973 gm/kg/3Y-C
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 138.17
2. Molar volume (cm3/mol): 334.1
3. Isotonic specific volume (90.2K): 1117.8
4. Surface tension (dyne/cm): 125.2
5. Polarizability (10-24cm3): 54.77
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -1.3
2. Number of hydrogen bond donors: 10
3. Number of hydrogen bond acceptors: 16
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: 162
6. Topological molecular polar surface area 266
7. Number of heavy atoms: 43
8. Surface charge: 0
9. Complexity: 1020
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 10
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
2. Incompatible materials: strong oxidizing agents
Storage method
1. Seal and package in a kraft paper bag wrapped in a food-grade plastic bag, or in a glass bottle. Store in a cool and dry place. Do not store together with toxic chemicals.
Synthesis method
1. Rutin exists in rue leaves, tobacco leaves, orange peels, tomatoes, etc., and is particularly rich in sophora rice and buckwheat flowers. Rutin used medicinally in my country is extracted from Sophora japonica rice. After washing the Sophora japonica rice, boil it with hot water and milk of lime (to adjust the pH to 7.5-8) for 20 minutes, add borax, keep it at 95-100°C for half an hour, filter it while hot, and adjust the filtrate to pH 6-7 with hydrochloric acid. Cool to below 30°C, let it stand to precipitate crystals, and separate to obtain crude rutin. After dissolving, recrystallizing, and drying, the finished product is obtained. The total yield based on Sophora japonica rice is 8.3%.
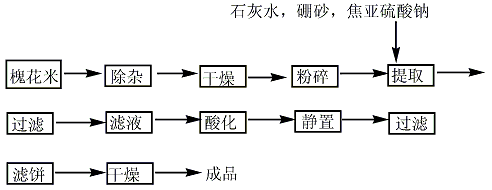
2. Combine the raw materials (usually Sophora buds) are crushed, extracted with hot ethanol, and then refined through multiple crystallizations of diethyl ether, hot methanol and hot water.
The residue obtained after the extract is concentrated is used to remove impurities such as other soluble pigments with a solvent, and then is crystallized multiple times with ethanol, ether, hot methanol and hot water and refined with activated carbon to obtain a high purity product.
Purpose
1. Can be used as edible antioxidants and nutritional enhancers.
2.Rutin has vitamin P-like effects and anti-inflammatory effects. It can reduce inflammation caused by mustard oil on the eyes or skin of animals, and has anti-inflammatory effects. Virus effect, at a concentration of 200μg·ml-1, it has the greatest inhibitory effect on vesicular stomatitis virus. It has strong antioxidant properties. Adding rutin to the homogenate rich in lysosomes can inhibit the formation of lipid peroxides. It can strongly absorb ultraviolet rays between 280 and 335nm and can be used in sunscreen and whitening cosmetics.
3.It has the functions of maintaining blood vessel resistance, reducing its permeability, and reducing fragility. It can be used to prevent and treat diseases such as cerebral hemorrhage, hypertension, retinal hemorrhage, purpura and acute hemorrhagic nephritis.

 微信扫一扫打赏
微信扫一扫打赏

