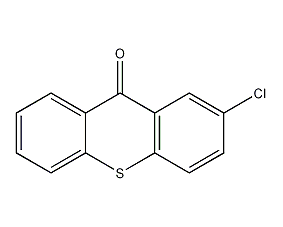
Structural formula
| Business number | 01WM |
|---|---|
| Molecular formula | C13H7ClOS |
| Molecular weight | 246.71 |
| label |
2-chlorothioxanth-9-one, 2-Chlorothioxanthen-9-one, 9H-Thioxanthen-9-one |
Numbering system
CAS number:86-39-5
MDL number:MFCD00005067
EINECS number:201-667-2
RTECS number:None
BRN number:None
PubChem number:24892945
Physical property data
1. Characteristics: yellow crystal. 2. Density (g/mL,25/4℃): Undetermined 3. Relative vapor density (g/mL, Air=1): Undetermined 4. Melting point (ºC):152.5-153.5 °C 5. Boiling point (ºC,Normal pressure): Undetermined 6. Boiling point (ºC,5.2kPa): Undetermined 7. Refractive index: Undetermined 8. Flashpoint (ºC):196 °F 9. Specific optical rotation (º): Undetermined 10. Autoignition point or ignition temperature (ºC): Undetermined 11. Vapor pressure (kPa,25ºC): Undetermined 12. Saturated vapor pressure (kPa,60ºC3. isotonic ratio (90.2K): 479.1 4. Surface tension (dyne/cm): 57.5 5. Polarizability(10-24 cm3):26.55
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 42.4
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 294
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored in a cool and dry place.
Synthesis method
It is obtained from anthranilic acid through diazotization, condensation and ring closure. First mix anthranilic acid, hydrochloric acid, and sodium nitrite at 0-5℃Carry out diazotization reaction to obtain a diazonium salt solution; then perform a condensation reaction with an alkali solution of p-chlorothiophenol, and acidify with hydrochloric acid to obtain4-Chlorodiphenyl sulfide-2′Carboxylic acid, mix it with concentrated sulfuric acid in60-70℃Catalytic closed loop to obtain the finished product.
Purpose
Pharmaceutical intermediate, used in the synthesis of the tranquillizer Telden (Chlorprothixene,[113-59-7]).
ning: 0pt”>Carry out diazotization reaction to obtain a diazonium salt solution; then perform a condensation reaction with an alkali solution of p-chlorothiophenol, and acidify with hydrochloric acid to obtain4-Chlorodiphenyl sulfide-2′Carboxylic acid, mix it with concentrated sulfuric acid60-70℃Catalytic closed loop to obtain the finished product.
Purpose
Pharmaceutical intermediate, used in the synthesis of the tranquillizer Telden (Chlorprothixene,[113-59-7]).

 微信扫一扫打赏
微信扫一扫打赏

