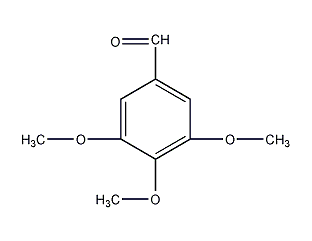
Structural formula
| Business number | 01X3 |
|---|---|
| Molecular formula | C10H12O4 |
| Molecular weight | 196.20 |
| label |
Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:86-81-7
MDL number:MFCD00003364
EINECS number:201-701-6
RTECS number:CU8462000
BRN number:395163
PubChem number:24889619
Physical property data
1. Properties: white to slightly yellow needle-like crystals
2. Melting point (℃): 74~75
Toxicological data
Rat caliber LD50:>33487mg/kg;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 53.04
2. Molar volume (cm3/mol): 173.1
3. Isotonic specific volume (90.2K): 422.3
4. Surface tension (dyne/cm): 35.4
5. Polarizability (10-24cm3): 21.02
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 44.8
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 169
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Requires production equipment to be airtight and the workshop to be well ventilated. Operators wear protective equipment.
Storage method
Protect from light and keep airtight.
Packed in buckets. Store in a cool, dry, ventilated warehouse and seal tightly. Store and transport according to general chemical regulations.
Synthesis method
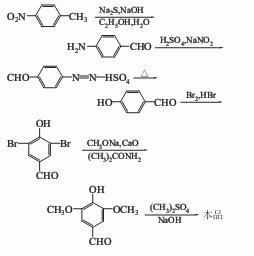
1. It is produced by methylating and esterifying gallic acid (or tannic acid) with dimethyl sulfate, then carrying out hydrazination reaction with hydrazine hydrate, and then oxidizing it with red blood salt. The yield of industrial production is above 70%.
2. Obtained from vanillic aldehyde through bromination, hydrolysis and methylation.
3. Use p-nitrotoluene as raw material, undergo oxidation and reduction, then diazotize and hydrolyze with sodium nitrite to obtain p-hydroxybenzaldehyde, which is then brominated, dimethoxylated and methylated And get. In addition, 3,4,5-trimethoxybenzaldehyde can also be prepared from trimethoxybenzonitrile and p-hydroxybenzaldehyde.
Purpose
Used in the synthesis of sulfa drugs, antibacterial synergist TMP and anti-epileptic drugs.

 微信扫一扫打赏
微信扫一扫打赏

