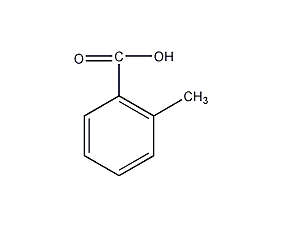
Structural formula
| Business number | 03AA |
|---|---|
| Molecular formula | C8H8O2 |
| Molecular weight | 136 |
| label |
2-methylbenzoic acid, 2-Methylbenzoic acid, o-Methylbenzoic acid, Pesticide intermediates; aromatic carboxylic acids and their derivatives, acidic solvent |
Numbering system
CAS number:118-90-1
MDL number:MFCD00002477
EINECS number:204-284-9
RTECS number:XU1400000
BRN number:1072103
PubChem ID:None
Physical property data
1. Properties: Prismatic crystals or needle-like crystals are precipitated from water.
2. Density (g/mL, 25℃): 1.062
3. Relative density (25℃, 4℃): 1.062115
4. Melting point (ºC): 103~105
5. Boiling point (ºC, normal pressure): 258.5751
6. Boiling point (ºC, 99.9kPa): 258~259
7. Refractive index (n115D): 1.512
8. Flash point (ºC): 148
9. Refractive index at room temperature (n25): 1.512115
10. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3971.29
11. Gas phase standard claimed heat (enthalpy) (kJ ·mol-1): -302.12
12. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -3874.59
13. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -416.52
14. Critical temperature (ºC): Not available Determined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17 . Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water , easily soluble in ethanol and chloroform.
Toxicological data
1. Acute toxicity: Rat oral LD50: 400mg/kg
Rabbit dermal LD50: 750mg/kg
Ecological data
This substance is harmful to the environment and can cause pollution to water bodies and the atmosphere. Organic acids can easily form acid rain during atmospheric chemistry and atmospheric physical changes. Therefore
When the pH value drops below 5, it will cause serious harm to animals and plants, and the reproduction and development of fish will be seriously affected. The metals can be dissolved into the water and poison fish. Acidification of water bodies will also lead to changes in the composition and structure of aquatic life. Acid-resistant algae and fungi will increase, while root plants, bacteria and vertebrates will decrease, and organic matter will decrease.The decomposition rate of �� is reduced. Acidification will seriously lead to the reduction or death of fish species in lakes and rivers.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 38.00
2. Molar volume (cm3/mol): 118.2
3. Isotonic specific volume (90.2K): 307.0
4. Surface tension (dyne/cm): 45.4
5. Polarizability (10-24cm3): 15.06
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.5
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 37.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 131
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Quantity: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides. Toxicity of this product: oral LD50400mg/kg in rats. Mice were intraperitoneally injected with LD50 420mg/kg. See benzoic acid.
2. Found in burley tobacco leaves.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Store away from oxidants, fire sources, and heat sources.
Packaging is in plastic woven bags. Store and transport according to general chemical regulations. See benzoic acid.
Synthesis method
1. Obtained from catalytic oxidation of o-xylene. Using o-xylene as raw material and cobalt naphthenate as catalyst, at a temperature of 120°C and a pressure of 0.245MPa, o-xylene continuously enters the oxidation tower for air oxidation, and the oxidation liquid enters the stripping tower for concentration, crystallization, and centrifugation to obtain Finished product. The mother liquor is distilled, o-xylene and part of o-toluic acid are recovered, and then the slag is discharged. The yield is 74%. Each ton of product consumes 1,300kg of o-xylene (95%).
The preparation method is to use o-xylene in the presence of cobalt naphthenate catalyst
in an oxidation tower at a reaction temperature of 120-125°C and a pressure of 196-392kPa. Continuous oxidation with air can produce finished products.
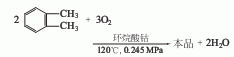
2. Tobacco: BU, 26.
Purpose
It is used as an organic synthesis intermediate for pesticides, fungicides, phosphoramides, spices, vinyl chloride polymerization initiator MBPO, m-cresol, color film developers, etc.

 微信扫一扫打赏
微信扫一扫打赏

