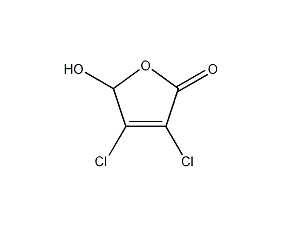
Structural formula
| Business number | 01Y4 |
|---|---|
| Molecular formula | C4H2Cl2O3 |
| Molecular weight | 168 |
| label |
2,3-Dichlorobutenoic acid, 2,3-Dichloromalealdehydic acid, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:87-56-9
MDL number:MFCD00006966
EINECS number:201-752-4
RTECS number:AS7700000
BRN number:1705641
PubChem number:24886036
Physical property data
1. Properties: white crystal or light yellow powder with pungent odor. [1]
2. Melting point (℃): 125~128[2]
3. Octanol/water Distribution coefficient: 1.37[3]
4. Flash point (℃): 100[4]
5. Dissolution Properties: Slightly soluble in water, soluble in hot water, hot benzene and ethanol. [5]
Toxicological data
1. Acute toxicity
Rat caliber LD50: 100mg/kg; mouse caliber TDLo: 84mg/kg; rabbit caliber TDLo: 160mg/kg; pig caliber TDLo: 100mg/kg;
p>
2. Neurotoxicity
Rabbit skin test: 20mg/24HREACTION SEVERITY;
Rabbit eye test: 50ug/24HREACTION SEVERITY;
3 , other multiple dose toxicity data
Rat caliber TDLo: 1218gm/kg/9W-C;
4. Chronic toxicity/carcinogenicity:
Mouse Caliber TDLo: 6100mg/kg/78W-I;
5. Teratogenicity
Salmonella: 100 ng/plate; Escherichia coli: 360 ug/L; Hamster ovary: 360 ug /L;
6. Acute toxicity[6] LD50: 50~100mg/kg (rat oral )
7. Irritation[7]
Rabbit transdermal: 2 mg ( 24h), severe stimulation.
Rabbit eye: 50μg (24h), severe irritation.
8. Subacute and chronic toxicity[8]
Repeatedly use higher concentrations Applying it to the skin of guinea pigs can reduce the number of hemoglobin and red blood cells, and cause weight loss.
9. Mutagenicity[9]
Microbial mutagenicity: Salmonella typhimurium Bacteria 100ng/dish. Mammalian somatic mutations: hamster ovary 23700 nmol/L.
Ecological data
1. Ecotoxicity[10]
LC50: 100~220mg/L (96h) (high body yarrow, static)
2. Biodegradability No information available
3. Non-biodegradability No information
Molecular structure data
1. Molar refractive index: 31.00
2. Molar volume (cm3/mol): 94.1
3. Isotonic specific volume (90.2K ): 259.0
4. Surface tension (dyne/cm): 57.2
5. Polarizability (10-24cm3): 12.29
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Topological molecular polar surface area (TPSA): 54.4
6. Number of heavy atoms: 9
7. Surface charge: 0
8. Complexity: 173
9. Isotopic atoms Quantity: 0
10. Determine the number of atomic stereocenters: 0
11. Uncertain number of atomic stereocenters: 0
12. Determine chemical bond positions Number of stereocenters: 1
13. Number of uncertain chemical bond stereocenters: 0
14. Number of covalent bond units: 1
Properties and stability
1. Stability[11] Stable
2. Incompatible substances[12] Strong oxidants, alkalis
3. Conditions to avoid contact[13] Heat
4. Polymerization hazard[14] Polymerization
5. Decomposition products[15] Hydrogen chloride
Storage method
Storage Precautions[16] Store in a cool, well-ventilated special warehouse, and implement the “two people to send and receive, and two people to keep” system. Keep away from fire and heat sources. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Furfural oxidative chlorination method: Furfural is obtained by passing chlorine gas into a strong acidic solution for oxidation and chlorination.

2. Manganese dioxide-hydrochloric acid Oxidative chlorination method: In the presence of hydrochloric acid, furfural and manganese dioxide are oxidized at low temperature, and then the temperature is increased for chlorination.
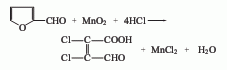
Purpose
1. Organic synthesis intermediates. It is used in the synthesis of broad-spectrum and highly efficient new acaricide pyridafenone and the herbicide benazone. It is also used in the synthesis of medicines such as sulfapyridine and photosensitive materials.
2. Used in organic synthesis and pharmaceuticals. [17]

 微信扫一扫打赏
微信扫一扫打赏

